Targeting soluble tumor necrosis factor as a potential intervention to lower risk for late-onset Alzheimer's disease associated with obesity, metabolic syndrome, and type 2 diabetes
- PMID: 31892368
- PMCID: PMC6937979
- DOI: 10.1186/s13195-019-0546-4
Targeting soluble tumor necrosis factor as a potential intervention to lower risk for late-onset Alzheimer's disease associated with obesity, metabolic syndrome, and type 2 diabetes
Abstract
Background: Insulin impairment and inflammation are two features common to type 2 diabetes and Alzheimer's disease; however, the molecular and signaling interactions underlying this relationship are not well understood. Mounting evidence point to the associations between the disruption of metabolite processing in insulin impairment and neurodegenerative conditions such as Alzheimer's. Although the brain depends partially on metabolites processed in the periphery, to date, little is known about how soluble tumor necrosis factor signaling (solTNF) impacts integrated peripheral immune and metabolic feedback signals in states of energy overload and insulin insensitivity.
Methods: C57Bl/6J mice were fed a high-fat high-carbohydrate diet (HFHC) for 14 weeks. The brain-permeant biologic XPro1595® was used to block solTNF-dependent pathways. Metabolic and immune alterations were evaluated in the gut, liver, and brain. Behavioral tests were performed. Untargeted metabolomics was carried out in the plasma and liver.
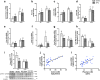
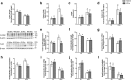
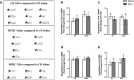
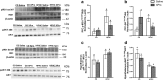
Results: HFHC diet promotes central insulin impairment and dysregulation of immune-modulatory gene expressed in the brain. Alteration of metabolites associated with type 2 diabetes and Alzheimer's such as butanoate, glutamate, biopterin, branched-chain amino acids, purines, and proteoglycan metabolism was observed in HFHC-fed mice. solTNF inhibition ameliorates hepatic metabolic disturbances and hepatic and intestinal lipocalin-2 levels, and decreases insulin impairment in the brain and behavioral deficits associated with HFHC diet.
Conclusions: Our novel findings suggest that HFHC diet impacts central insulin signaling and immune-metabolic interactions in a solTNF-dependent manner to increase the risk for neurodegenerative conditions. Our novel findings indicate that selective solTNF neutralization can ameliorate peripheral and central diet-induced insulin impairment and identify lipocalin-2 as a potential target for therapeutic intervention to target inflammation and insulin disturbances in obesogenic environments. Collectively, our findings identify solTNF as a potential target for therapeutic intervention in inflammatory states and insulin disturbances in obesogenic environments to lower risk for AD.
Keywords: Gut; Insulin; Lipocalin-2; Liver; Metabolic inflammation; Metabolomics; Neuroactive metabolites; Proteoglycans; Purines; Soluble tumor necrosis factor.
Conflict of interest statement
MG Tansey is an ex-employee of Xencor, Inc. and is a co-inventor on the dominant-negative TNF patent but does not hold a significant financial stake in Xencor, Inc. or INmune Bio, Inc. CJ Barnum is an employee of INmune Bio. The other authors declare that they have no competing interests.
Figures


Similar articles
-
Diet-induced metabolic and immune impairments are sex-specifically modulated by soluble TNF signaling in the 5xFAD mouse model of Alzheimer's disease.Neurobiol Dis. 2024 Jun 15;196:106511. doi: 10.1016/j.nbd.2024.106511. Epub 2024 Apr 24. Neurobiol Dis. 2024. PMID: 38670277
-
Diet-induced metabolic and immune impairments are sex-specifically modulated by soluble TNF signaling in the 5xFAD mouse model of Alzheimer's disease.bioRxiv [Preprint]. 2024 Feb 28:2024.02.28.582516. doi: 10.1101/2024.02.28.582516. bioRxiv. 2024. Update in: Neurobiol Dis. 2024 Jun 15;196:106511. doi: 10.1016/j.nbd.2024.106511. PMID: 38464096 Free PMC article. Updated. Preprint.
-
Chronic psychological stress and high-fat high-fructose diet disrupt metabolic and inflammatory gene networks in the brain, liver, and gut and promote behavioral deficits in mice.Brain Behav Immun. 2017 Jan;59:158-172. doi: 10.1016/j.bbi.2016.08.021. Epub 2016 Sep 2. Brain Behav Immun. 2017. PMID: 27592562 Free PMC article.
-
Alzheimer's disease and type 2 diabetes: multiple mechanisms contribute to interactions.Curr Diab Rep. 2014 Apr;14(4):476. doi: 10.1007/s11892-014-0476-2. Curr Diab Rep. 2014. PMID: 24526623 Free PMC article. Review.
-
Shared Neuropathological Characteristics of Obesity, Type 2 Diabetes and Alzheimer's Disease: Impacts on Cognitive Decline.Nutrients. 2015 Sep 1;7(9):7332-57. doi: 10.3390/nu7095341. Nutrients. 2015. PMID: 26340637 Free PMC article. Review.
Cited by
-
Identifying Proteomic Prognostic Markers for Alzheimer's Disease with Survival Machine Learning: the Framingham Heart Study.medRxiv [Preprint]. 2024 Sep 23:2024.09.21.24314123. doi: 10.1101/2024.09.21.24314123. medRxiv. 2024. Update in: J Prev Alzheimers Dis. 2025 Feb;12(2):100021. doi: 10.1016/j.tjpad.2024.100021. PMID: 39399041 Free PMC article. Updated. Preprint.
-
Insulin resistance: a connecting link between Alzheimer's disease and metabolic disorder.Metab Brain Dis. 2021 Jan;36(1):67-83. doi: 10.1007/s11011-020-00622-2. Epub 2020 Sep 28. Metab Brain Dis. 2021. PMID: 32986168 Review.
-
Soluble TNF mediates amyloid-independent, diet-induced alterations to immune and neuronal functions in an Alzheimer's disease mouse model.Front Cell Neurosci. 2023 Mar 15;17:895017. doi: 10.3389/fncel.2023.895017. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37006470 Free PMC article.
-
Identifying proteomic prognostic markers for Alzheimer's disease with survival machine learning: The Framingham Heart Study.J Prev Alzheimers Dis. 2025 Feb;12(2):100021. doi: 10.1016/j.tjpad.2024.100021. Epub 2025 Jan 1. J Prev Alzheimers Dis. 2025. PMID: 39863332 Free PMC article.
-
Common Protective Strategies in Neurodegenerative Disease: Focusing on Risk Factors to Target the Cellular Redox System.Oxid Med Cell Longev. 2020 Aug 1;2020:8363245. doi: 10.1155/2020/8363245. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32832006 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

