Proteome-wide observation of the phenomenon of life on the edge of solubility
- PMID: 31892536
- PMCID: PMC6969518
- DOI: 10.1073/pnas.1910444117
Proteome-wide observation of the phenomenon of life on the edge of solubility
Abstract
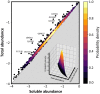
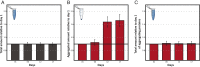
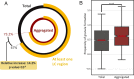
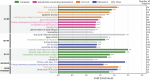
To function effectively proteins must avoid aberrant aggregation, and hence they are expected to be expressed at concentrations safely below their solubility limits. By analyzing proteome-wide mass spectrometry data of Caenorhabditis elegans, however, we show that the levels of about three-quarters of the nearly 4,000 proteins analyzed in adult animals are close to their intrinsic solubility limits, indeed exceeding them by about 10% on average. We next asked how aging and functional self-assembly influence these solubility limits. We found that despite the fact that the total quantity of proteins within the cellular environment remains approximately constant during aging, protein aggregation sharply increases between days 6 and 12 of adulthood, after the worms have reproduced, as individual proteins lose their stoichiometric balances and the cellular machinery that maintains solubility undergoes functional decline. These findings reveal that these proteins are highly prone to undergoing concentration-dependent phase separation, which on aging is rationalized in a decrease of their effective solubilities, in particular for proteins associated with translation, growth, reproduction, and the chaperone system.
Keywords: protein aggregation; protein homeostasis; protein misfolding diseases.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Patterson C., World Alzheimer Report 2018 (Alzheimer’s Disease International, London, UK, 2018).
-
- Chiti F., Dobson C. M., Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 86, 27–68 (2017). - PubMed
-
- De Strooper B., Karran E., The cellular phase of Alzheimer’s disease. Cell 164, 603–615 (2016). - PubMed
-
- Dobson C. M., Protein folding and misfolding. Nature 426, 884–890 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

