Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes
- PMID: 31892541
- PMCID: PMC6983422
- DOI: 10.1073/pnas.1914742117
Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes
Abstract
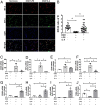
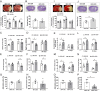
Microglial activation plays a central role in poststroke inflammation and causes secondary neuronal damage; however, it also contributes in debris clearance and chronic recovery. Microglial pro- and antiinflammatory responses (or so-called M1-M2 phenotypes) coexist and antagonize each other throughout the disease progress. As a result of this balance, poststroke immune responses alter stroke outcomes. Our previous study found microglial expression of interferon regulatory factor 5 (IRF5) and IRF4 was related to pro- and antiinflammatory responses, respectively. In the present study, we genetically modified the IRF5 and IRF4 signaling to explore their roles in stroke. Both in vitro and in vivo assays were utilized; IRF5 or IRF4 small interfering RNA (siRNA), lentivirus, and conditional knockout (CKO) techniques were employed to modulate IRF5 or IRF4 expression in microglia. We used a transient middle cerebral artery occlusion model to induce stroke and examined both acute and chronic stroke outcomes. Poststroke inflammation was evaluated with flow cytometry, RT-PCR, MultiPlex, and immunofluorescence staining. An oscillating pattern of the IRF5-IRF4 regulatory axis function was revealed. Down-regulation of IRF5 signaling by siRNA or CKO resulted in increased IRF4 expression, enhanced M2 activation, quenched proinflammatory responses, and improved stroke outcomes, whereas down-regulation of IRF4 led to increased IRF5 expression, enhanced M1 activation, exacerbated proinflammatory responses, and worse functional recovery. Up-regulation of IRF4 or IRF5 by lentivirus induced similar results. We conclude that the IRF5-IRF4 regulatory axis is a key determinant in microglial activation. The IRF5-IRF4 regulatory axis is a potential therapeutic target for neuroinflammation and ischemic stroke.
Keywords: brain; interferon regulatory factor; microglia; neuroinflammation; stroke.
Conflict of interest statement
The authors declare no competing interest.
Figures






References
-
- Hu X., et al. , Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43, 3063–3070 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

