Intraoperative assessment of skull base tumors using stimulated Raman scattering microscopy
- PMID: 31892723
- PMCID: PMC6938502
- DOI: 10.1038/s41598-019-56932-8
Intraoperative assessment of skull base tumors using stimulated Raman scattering microscopy
Abstract
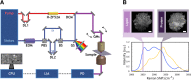
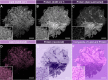
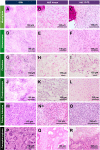
Intraoperative consultations, used to guide tumor resection, can present histopathological findings that are challenging to interpret due to artefacts from tissue cryosectioning and conventional staining. Stimulated Raman histology (SRH), a label-free imaging technique for unprocessed biospecimens, has demonstrated promise in a limited subset of tumors. Here, we target unexplored skull base tumors using a fast simultaneous two-channel stimulated Raman scattering (SRS) imaging technique and a new pseudo-hematoxylin and eosin (H&E) recoloring methodology. To quantitatively evaluate the efficacy of our approach, we use modularized assessment of diagnostic accuracy beyond cancer/non-cancer determination and neuropathologist confidence for SRH images contrasted to H&E-stained frozen and formalin-fixed paraffin-embedded (FFPE) tissue sections. Our results reveal that SRH is effective for establishing a diagnosis using fresh tissue in most cases with 87% accuracy relative to H&E-stained FFPE sections. Further analysis of discrepant case interpretation suggests that pseudo-H&E recoloring underutilizes the rich chemical information offered by SRS imaging, and an improved diagnosis can be achieved if full SRS information is used. In summary, our findings show that pseudo-H&E recolored SRS images in combination with lipid and protein chemical information can maximize the use of SRS during intraoperative pathologic consultation with implications for tissue preservation and augmented diagnostic utility.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Powell SZ. Intraoperative Consultation, Cytologic Preparations, and Frozen Section in the Central Nervous System. Arch Pathol Lab Med. 2005;129:18. - PubMed
-
- Louis, D., Ohgaki, H., Wiestler, O. & Cavenee, W. (eds) WHO Classification of Tumours of the Central Nervous System, Fourth Edition fourth edn, (World Health Organization and International Agency for Research on Cancer, 2007).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

