Legumain Promotes Gastric Cancer Progression Through Tumor-associated Macrophages In vitro and In vivo
- PMID: 31892854
- PMCID: PMC6930372
- DOI: 10.7150/ijbs.36467
Legumain Promotes Gastric Cancer Progression Through Tumor-associated Macrophages In vitro and In vivo
Abstract
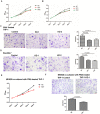
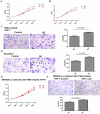
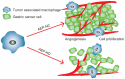
Tumor-associated macrophages (TAMs) play a crucial role in the tumor microenvironment. Legumain (LGMN) has been shown to be a tumor-promoting protein, but the effect of LGMN on TAMs in the progression of gastric cancer (GC) is under exploration. Our studies included the construction of LGMN-knockdown and LGMN-overexpressing TAMs induced from the human cell line THP-1 (PMA/IL-4/IL-13) and murine cell line Raw264.7 (IL-4/IL-13). A CCK-8 assay and transwell migration assay indicated that upregulation of LGMN expression in TAMs stimulated cell proliferation, migration and invasion in vitro, while downregulation of LGMN expression reduced cell proliferation, migration and invasion. In vivo experiments revealed slower growth, less angiogenesis, and less Ki67 expression in LGMN-knockdown TAMs injected with gastric cancer cells compared to control TAMs injected with GC cells. Together, these study results suggested that LGMN+ TAMs, which may serve as a potential target for GC treatment, promoted gastric cancer cell proliferation and angiogenesis in vitro and in vivo.
Keywords: Legumain; angiogenesis; gastric cancer; tumor-associated macrophages.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
An Overview of Targeting Legumain for Inhibiting Cancers.Curr Pharm Des. 2021 Oct 5;27(31):3337-3348. doi: 10.2174/1381612826666201125111625. Curr Pharm Des. 2021. PMID: 33238867 Review.
-
Bu Fei Decoction attenuates the tumor associated macrophage stimulated proliferation, migration, invasion and immunosuppression of non-small cell lung cancer, partially via IL-10 and PD-L1 regulation.Int J Oncol. 2017 Jul;51(1):25-38. doi: 10.3892/ijo.2017.4014. Epub 2017 May 19. Int J Oncol. 2017. PMID: 28534943 Free PMC article.
-
Tumor-associated macrophages induce the expression of FOXQ1 to promote epithelial-mesenchymal transition and metastasis in gastric cancer cells.Oncol Rep. 2017 Oct;38(4):2003-2010. doi: 10.3892/or.2017.5877. Epub 2017 Aug 3. Oncol Rep. 2017. PMID: 28791370 Free PMC article.
-
Role of LGMN in tumor development and its progression and connection with the tumor microenvironment.Front Mol Biosci. 2023 Feb 7;10:1121964. doi: 10.3389/fmolb.2023.1121964. eCollection 2023. Front Mol Biosci. 2023. PMID: 36825203 Free PMC article. Review.
-
LncRNA-MALAT1 Promotes Angiogenesis of Thyroid Cancer by Modulating Tumor-Associated Macrophage FGF2 Protein Secretion.J Cell Biochem. 2017 Dec;118(12):4821-4830. doi: 10.1002/jcb.26153. Epub 2017 Jun 13. J Cell Biochem. 2017. PMID: 28543663
Cited by
-
An infection-induced oxidation site regulates legumain processing and tumor growth.Nat Chem Biol. 2022 Jul;18(7):698-705. doi: 10.1038/s41589-022-00992-x. Epub 2022 Mar 24. Nat Chem Biol. 2022. PMID: 35332331 Free PMC article.
-
Circadian Regulator CLOCK Drives Immunosuppression in Glioblastoma.Cancer Immunol Res. 2022 Jun 3;10(6):770-784. doi: 10.1158/2326-6066.CIR-21-0559. Cancer Immunol Res. 2022. PMID: 35413115 Free PMC article.
-
Extracellular vesicles in endometriosis: role and potential.Front Endocrinol (Lausanne). 2024 Apr 26;15:1365327. doi: 10.3389/fendo.2024.1365327. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38737555 Free PMC article. Review.
-
Oxidative Stress and Redox Signaling in Gastric Cancer: From Mechanisms to Therapeutic Implications.Antioxidants (Basel). 2025 Feb 24;14(3):258. doi: 10.3390/antiox14030258. Antioxidants (Basel). 2025. PMID: 40227215 Free PMC article. Review.
-
Legumain in cardiovascular diseases.Exp Biol Med (Maywood). 2024 Jul 22;249:10121. doi: 10.3389/ebm.2024.10121. eCollection 2024. Exp Biol Med (Maywood). 2024. PMID: 39104790 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67:7–30. - PubMed
-
- Salomon JA, Wang H, Freeman MK, Vos T, Flaxman AD, Lopez AD. et al. Healthy life expectancy for 187 countries, 1990-2010: a systematic analysis for the Global Burden Disease Study 2010. Lancet (London, England) 2012;380:2144–62. - PubMed
-
- Zhang X, Li M, Chen S, Hu J, Guo Q, Liu R. et al. Endoscopic Screening in Asian Countries Is Associated With Reduced Gastric Cancer Mortality: A Meta-analysis and Systematic Review. Gastroenterology. 2018;155:347–54.e9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

