Cysteine catabolism and the serine biosynthesis pathway support pyruvate production during pyruvate kinase knockdown in pancreatic cancer cells
- PMID: 31893043
- PMCID: PMC6937848
- DOI: 10.1186/s40170-019-0205-z
Cysteine catabolism and the serine biosynthesis pathway support pyruvate production during pyruvate kinase knockdown in pancreatic cancer cells
Abstract
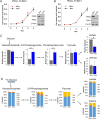
Background: Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer with limited treatment options. Pyruvate kinase, especially the M2 isoform (PKM2), is highly expressed in PDAC cells, but its role in pancreatic cancer remains controversial. To investigate the role of pyruvate kinase in pancreatic cancer, we knocked down PKM2 individually as well as both PKM1 and PKM2 concurrently (PKM1/2) in cell lines derived from a Kras G12D/- ; p53 -/- pancreatic mouse model.
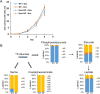
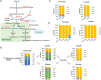
Methods: We used liquid chromatography tandem mass spectrometry (LC-MS/MS) to determine metabolic profiles of wildtype and PKM1/2 knockdown PDAC cells. We further used stable isotope-labeled metabolic precursors and LC-MS/MS to determine metabolic pathways upregulated in PKM1/2 knockdown cells. We then targeted metabolic pathways upregulated in PKM1/2 knockdown cells using CRISPR/Cas9 gene editing technology.
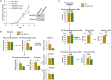
Results: PDAC cells are able to proliferate and continue to produce pyruvate despite PKM1/2 knockdown. The serine biosynthesis pathway partially contributed to pyruvate production during PKM1/2 knockdown: knockout of phosphoglycerate dehydrogenase in this pathway decreased pyruvate production from glucose. In addition, cysteine catabolism generated ~ 20% of intracellular pyruvate in PDAC cells. Other potential sources of pyruvate include the sialic acid pathway and catabolism of glutamine, serine, tryptophan, and threonine. However, these sources did not provide significant levels of pyruvate in PKM1/2 knockdown cells.
Conclusion: PKM1/2 knockdown does not impact the proliferation of pancreatic cancer cells. The serine biosynthesis pathway supports conversion of glucose to pyruvate during pyruvate kinase knockdown. However, direct conversion of serine to pyruvate was not observed during PKM1/2 knockdown. Investigating several alternative sources of pyruvate identified cysteine catabolism for pyruvate production during PKM1/2 knockdown. Surprisingly, we find that a large percentage of intracellular pyruvate comes from cysteine. Our results highlight the ability of PDAC cells to adaptively rewire their metabolic pathways during knockdown of a key metabolic enzyme.
Keywords: Liquid chromatography mass spectrometry; Metabolism; PKM; Pancreatic cancer; Pyruvate kinase.
© The Author(s). 2019.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures

Similar articles
-
Glucose Catabolism in Liver Tumors Induced by c-MYC Can Be Sustained by Various PKM1/PKM2 Ratios and Pyruvate Kinase Activities.Cancer Res. 2017 Aug 15;77(16):4355-4364. doi: 10.1158/0008-5472.CAN-17-0498. Epub 2017 Jun 19. Cancer Res. 2017. PMID: 28630053 Free PMC article.
-
Pyruvate kinase M2 promotes pancreatic ductal adenocarcinoma invasion and metastasis through phosphorylation and stabilization of PAK2 protein.Oncogene. 2018 Mar;37(13):1730-1742. doi: 10.1038/s41388-017-0086-y. Epub 2018 Jan 16. Oncogene. 2018. PMID: 29335522
-
Pyruvate Kinase Activity Regulates Cystine Starvation Induced Ferroptosis through Malic Enzyme 1 in Pancreatic Cancer Cells.bioRxiv [Preprint]. 2023 Sep 17:2023.09.15.557984. doi: 10.1101/2023.09.15.557984. bioRxiv. 2023. PMID: 37745559 Free PMC article. Preprint.
-
Pyruvate Kinase M2 and Cancer: The Role of PKM2 in Promoting Tumorigenesis.Front Oncol. 2020 Mar 2;10:159. doi: 10.3389/fonc.2020.00159. eCollection 2020. Front Oncol. 2020. PMID: 32195169 Free PMC article. Review.
-
Metabolic pathway alterations that support cell proliferation.Cold Spring Harb Symp Quant Biol. 2011;76:325-34. doi: 10.1101/sqb.2012.76.010900. Epub 2012 Jan 19. Cold Spring Harb Symp Quant Biol. 2011. PMID: 22262476 Review.
Cited by
-
Overcoming chemoresistance by targeting reprogrammed metabolism: the Achilles' heel of pancreatic ductal adenocarcinoma.Cell Mol Life Sci. 2021 Jul;78(14):5505-5526. doi: 10.1007/s00018-021-03866-y. Epub 2021 Jun 15. Cell Mol Life Sci. 2021. PMID: 34131808 Free PMC article. Review.
-
Development of a Novel Benzimidazole-Based Probe and Portable Fluorimeter for the Detection of Cysteine in Human Urine.Biosensors (Basel). 2021 Oct 26;11(11):420. doi: 10.3390/bios11110420. Biosensors (Basel). 2021. PMID: 34821635 Free PMC article.
-
From Glucose to Lactate and Transiting Intermediates Through Mitochondria, Bypassing Pyruvate Kinase: Considerations for Cells Exhibiting Dimeric PKM2 or Otherwise Inhibited Kinase Activity.Front Physiol. 2020 Dec 1;11:543564. doi: 10.3389/fphys.2020.543564. eCollection 2020. Front Physiol. 2020. PMID: 33335484 Free PMC article. Review.
-
New insights into molecules and pathways of cancer metabolism and therapeutic implications.Cancer Commun (Lond). 2021 Jan;41(1):16-36. doi: 10.1002/cac2.12112. Epub 2020 Nov 10. Cancer Commun (Lond). 2021. PMID: 33174400 Free PMC article. Review.
-
O-GlcNAc signaling increases neuron regeneration through one-carbon metabolism in Caenorhabditis elegans.Elife. 2024 Feb 9;13:e86478. doi: 10.7554/eLife.86478. Elife. 2024. PMID: 38334260 Free PMC article.
References
-
- 5-year relative survival, 2008-2014, cancer statistic center, American Cancer Society. https://cancerstatisticscenter.cancer.org/#/
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

