Complement activation in individuals with previous subclinical Lyme borreliosis and patients with previous Lyme neuroborreliosis
- PMID: 31893341
- PMCID: PMC7182544
- DOI: 10.1007/s10096-019-03807-5
Complement activation in individuals with previous subclinical Lyme borreliosis and patients with previous Lyme neuroborreliosis
Abstract
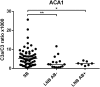
Lyme borreliosis (LB) is caused by Borrelia burgdorferi and infection may lead to not only a large variety of clinical manifestations but also a subclinical outcome. The aim of the present study was to investigate if there is a constitutional difference in complement activation between individuals with previous subclinical Lyme borreliosis (SB) and patients previously diagnosed with Lyme neuroborreliosis (LNB).Lepirudin plasma for activation studies was collected from 60 SB individuals and from 22 patients pre-diagnosed with LNB. The plasma was incubated with live Borrelia spirochetes of two strains (complement sensitive B. garinii Lu59 and complement resistant B. afzelii ACA1).Complement factor C3 was measured in non-activated lepirudin plasma with immune-nephelometry and C3a and sC5b-9 generated during complement activation were measured by enzyme-linked immunosorbent assay.We found that the complement sensitive Lu59 induced higher complement activation than the complement resistant ACA1 when measuring activation products C3a and sC5b-9 in SB and LNB patients, p < 0.0001. No significant difference was found between SB and LNB patients in systemic levels of C3. Furthermore, SB individuals generated a higher activation of C3 cleavage to C3a (C3a/C3 ratio) than LNB patients after activation with ACA1, p < 0.001, but no significant differences were found in response to Lu59. In conclusion, Lu59 induced higher complement activation than ACA1 and individuals with previous SB showed increased generation of C3a compared with patients with previous LNB. In our study population, this mechanism could lead to less elimination of spirochetes in LNB patients and thereby be a factor contributing to the clinical outcome.
Keywords: C3a; Complement activation; Innate immune system; Lyme borreliosis; Lyme neuroborreliosis; Subclinical Lyme borreliosis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Oosting M, Berende A, Sturm P, Ter Hofstede HJ, de Jong DJ, Kanneganti TD, van der Meer JW, Kullberg BJ, Netea MG, Joosten LA. Recognition of Borrelia burgdorferi by NOD2 is central for the induction of an inflammatory reaction. J Infect Dis. 2010;201(12):1849–1858. doi: 10.1086/652871. - DOI - PubMed
-
- Skogman BH, Hellberg S, Ekerfelt C, Jenmalm MC, Forsberg P, Ludvigsson J, Bergstrom S, Ernerudh J. Adaptive and innate immune responsiveness to Borrelia burgdorferi sensu lato in exposed asymptomatic children and children with previous clinical Lyme borreliosis. Clin Dev Immunol. 2012;2012:294587. doi: 10.1155/2012/294587. - DOI - PMC - PubMed
-
- Wilhelmsson P, Fryland L, Lindblom P, Sjowall J, Ahlm C, Berglund J, Haglund M, Henningsson AJ, Nolskog P, Nordberg M, Nyberg C, Ornstein K, Nyman D, Ekerfelt C, Forsberg P, Lindgren PE. A prospective study on the incidence of Borrelia burgdorferi sensu lato infection after a tick bite in Sweden and on the Aland Islands, Finland (2008-2009) Ticks Tick Borne Dis. 2016;7(1):71–79. doi: 10.1016/j.ttbdis.2015.08.009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

