Development of therapeutic antibodies for the treatment of diseases
- PMID: 31894001
- PMCID: PMC6939334
- DOI: 10.1186/s12929-019-0592-z
Development of therapeutic antibodies for the treatment of diseases
Abstract
It has been more than three decades since the first monoclonal antibody was approved by the United States Food and Drug Administration (US FDA) in 1986, and during this time, antibody engineering has dramatically evolved. Current antibody drugs have increasingly fewer adverse effects due to their high specificity. As a result, therapeutic antibodies have become the predominant class of new drugs developed in recent years. Over the past five years, antibodies have become the best-selling drugs in the pharmaceutical market, and in 2018, eight of the top ten bestselling drugs worldwide were biologics. The global therapeutic monoclonal antibody market was valued at approximately US$115.2 billion in 2018 and is expected to generate revenue of $150 billion by the end of 2019 and $300 billion by 2025. Thus, the market for therapeutic antibody drugs has experienced explosive growth as new drugs have been approved for treating various human diseases, including many cancers, autoimmune, metabolic and infectious diseases. As of December 2019, 79 therapeutic mAbs have been approved by the US FDA, but there is still significant growth potential. This review summarizes the latest market trends and outlines the preeminent antibody engineering technologies used in the development of therapeutic antibody drugs, such as humanization of monoclonal antibodies, phage display, the human antibody mouse, single B cell antibody technology, and affinity maturation. Finally, future applications and perspectives are also discussed.
Keywords: Affinity maturation; Antibody market; Human antibody mouse; Humanized antibody; Phage display; Single B cell antibody technology; Therapeutic antibody.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

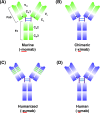

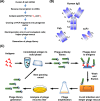
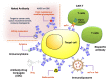
References
-
- The Antibody Society. In: Approved antibodies. Jun 27, 2019 https://www.antibodysociety.org/ Accessed 15 Jul 2019.
-
- Lefranc MP. IMGT, the International ImMunoGeneTics Information System. Cold Spring Harb Protoc. 2011;2011:595–603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

