Prospective longitudinal atrophy in Alzheimer's disease correlates with the intensity and topography of baseline tau-PET
- PMID: 31894103
- PMCID: PMC7035952
- DOI: 10.1126/scitranslmed.aau5732
Prospective longitudinal atrophy in Alzheimer's disease correlates with the intensity and topography of baseline tau-PET
Abstract
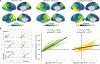
β-Amyloid plaques and tau-containing neurofibrillary tangles are the two neuropathological hallmarks of Alzheimer's disease (AD) and are thought to play crucial roles in a neurodegenerative cascade leading to dementia. Both lesions can now be visualized in vivo using positron emission tomography (PET) radiotracers, opening new opportunities to study disease mechanisms and improve patients' diagnostic and prognostic evaluation. In a group of 32 patients at early symptomatic AD stages, we tested whether β-amyloid and tau-PET could predict subsequent brain atrophy measured using longitudinal magnetic resonance imaging acquired at the time of PET and 15 months later. Quantitative analyses showed that the global intensity of tau-PET, but not β-amyloid-PET, signal predicted the rate of subsequent atrophy, independent of baseline cortical thickness. Additional investigations demonstrated that the specific distribution of tau-PET signal was a strong indicator of the topography of future atrophy at the single patient level and that the relationship between baseline tau-PET and subsequent atrophy was particularly strong in younger patients. These data support disease models in which tau pathology is a major driver of local neurodegeneration and highlight the relevance of tau-PET as a precision medicine tool to help predict individual patient's progression and design future clinical trials.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Competing interests
Renaud La Joie, Adrienne A. Visani, Jesse A. Brown, Viktoriya Bourakova, Jungho Cha, Kiran Chaudhary, Lauren Edwards, Leonardo Iaccarino, Mustafa Janabi, Orit Lesman-Segev, Zachary Miller, James P O’Neil and Julie Pham report no disclosure.
Figures




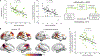
References
-
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ, National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease, Alzheimers Dement 8, 1–13 (2012). - PMC - PubMed
-
- Duyckaerts C, Delatour B, Potier M-C, Classification and basic pathology of Alzheimer disease, Acta Neuropathol. (Berl.) 118, 5–36 (2009). - PubMed
-
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang G, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B, Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B, Ann. Neurol 55, 306–319 (2004). - PubMed
-
- Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su M-Y, Shankle WR, Elizarov A, Kolb HC, Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807, J. Alzheimers Dis. JAD 34, 457–468 (2013). - PubMed
-
- Cho H, Choi JY, Hwang MS, Lee JH, Kim YJ, Lee HM, Lyoo CH, Ryu YH, Lee MS, Tau PET in Alzheimer disease and mild cognitive impairment, Neurology 87, 375–383 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K24 AG045333/AG/NIA NIH HHS/United States
- U01 AG057195/AG/NIA NIH HHS/United States
- R01 AG038791/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- R01 AG032306/AG/NIA NIH HHS/United States
- R01 AG045611/AG/NIA NIH HHS/United States
- K23 AG045289/AG/NIA NIH HHS/United States
- R01 AG034570/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- T32 AG023481/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- K01 AG055698/AG/NIA NIH HHS/United States
- K23 AG059888/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

