Frontiers in Halogen and Chalcogen-Bond Donor Organocatalysis
- PMID: 31894187
- PMCID: PMC6919929
- DOI: 10.1002/cctc.201901215
Frontiers in Halogen and Chalcogen-Bond Donor Organocatalysis
Abstract
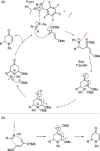
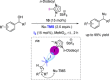
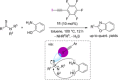
Non-covalent molecular interactions on the basis of halogen and chalcogen bonding represent a promising, powerful catalytic activation mode. However, these "unusual" non-covalent interactions are typically employed in the solid state and scarcely exploited in catalysis. In recent years, an increased interest in halogen and chalcogen bonding has been awaken, as they provide profound characteristics that make them an appealing alternative to the well-explored hydrogen bonding. Being particularly relevant in the binding of "soft" substrates, the similar strength to hydrogen bonding interactions and its higher directionality allows for solution-phase applications with halogen and chalcogen bonding as the key interaction. In this mini-review, the special features, state-of-the-art and key examples of these so-called σ-hole interactions in the field of organocatalysis are presented.
Keywords: Chalcogen; Halogen-bonding; Non-covalent activation; Organocatalysis; Sigma-hole.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

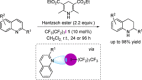
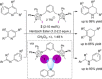
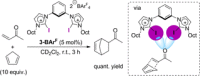
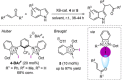
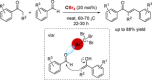
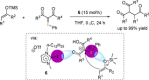
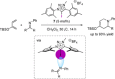
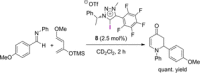
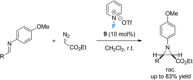
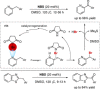
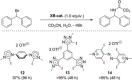
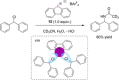
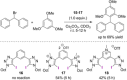
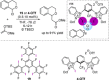
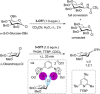
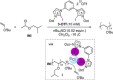
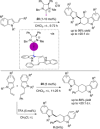


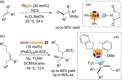
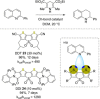
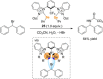

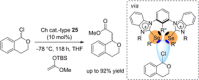
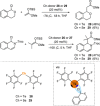
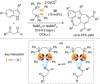
References
Publication types
LinkOut - more resources
Full Text Sources
