Delayed Denosumab Injections and Bone Mineral Density Response: An Electronic Health Record-based Study
- PMID: 31894244
- PMCID: PMC7089847
- DOI: 10.1210/clinem/dgz321
Delayed Denosumab Injections and Bone Mineral Density Response: An Electronic Health Record-based Study
Abstract
Context: Discontinuation of denosumab leads to a rapid reversal of its therapeutic effect. However, there are no data regarding how unintended delays or missed injections of denosumab impact bone mineral density (BMD) response.
Objective: We examined the association of delays in injections of denosumab with BMD change.
Design: We used electronic medical records from two academic hospitals from 2010 to 2017.
Participants: Patients older than 45 years of age and used at least 2 doses of 60 mg denosumab. Denosumab adherence was evaluated by the medication coverage ratio (MCR). Good adherence corresponds to a dosing interval ≤7 months (defined by MCR ≥93%), moderate adherence corresponds to an interval of 7 to 10 months (MCR 75%-93%), and poor adherence corresponds to an interval ≥10 months (MCR ≤75%).
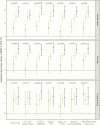
Outcome measures: Annualized percent BMD change from baseline at the lumbar spine, total hip, and femoral neck.
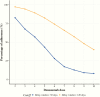
Results: We identified 938 denosumab injections among 151 patients; the mean (SD) age was 69 (10) years, and 95% were female. Patients with good adherence had an annualized BMD increase of 3.9% at the lumbar spine, compared with patients with moderate (3.0%) or poor adherence (1.4%, P for trend .002). Patients with good adherence had an annualized BMD increase of 2.1% at the total hip, compared with patients with moderate (1.3%) or poor adherence (0.6%, P for trend .002).
Conclusions: A longer interval between denosumab injections is associated with suboptimal BMD response at both spine and total hip. Strategies to improve the timely administration of denosumab in real-world settings are needed.
Keywords: Osteoporosis; bone mineral density; denosumab; dosing delay.
© Endocrine Society 2020. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Letter to the Editor: "Delayed Denosumab Injections and Bone Mineral Density Response: En Electronic Health Record-Based Study".J Clin Endocrinol Metab. 2020 Oct 1;105(10):dgaa384. doi: 10.1210/clinem/dgaa384. J Clin Endocrinol Metab. 2020. PMID: 32556183 No abstract available.
References
-
- Lacey DL, Boyle WJ, Simonet WS, et al. Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11(5): nrd3705. - PubMed
-
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96(4):972–980. - PubMed
-
- Cummings SR, San Martin J, McClung MR, et al. ; FREEDOM Trial Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8): 756–765. - PubMed
-
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–523. - PubMed
-
- Anastasilakis AD, Polyzos SA, Makras P. Therapy of endocrine disease: denosumab vs bisphosphonates for the treatment of postmenopausal osteoporosis. Eur J Endocrinol. 2018;179(1):R31–R45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

