Dexamethasone induces aberrant macrophage immune function and apoptosis
- PMID: 31894280
- PMCID: PMC6967116
- DOI: 10.3892/or.2019.7434
Dexamethasone induces aberrant macrophage immune function and apoptosis
Abstract
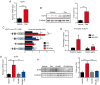
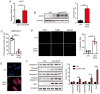
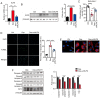
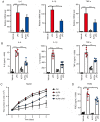
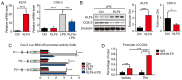
Glucocorticoids (GCs) are known potent clinical drugs, however, their mode of action is still complex and debatable. Macrophages are the most important target of GCs and play a key role in tumor immunity in vivo, but their relationship is also controversial. In the present study, the lentivirus system was used to overexpress and knock down the level of transcription factor Krüppel‑like factor 9 (KLF9). The results revealed that dexamethasone (Dex) induced ROS generation and mitochondria‑dependent apoptosis in RAW 264.7 cells via the KLF9. In addition, overexpression of KLF9 significantly increased apoptosis of RAW 264.7 cells. Notably, ELISA assay revealed that increased expression of KLF9 inhibited LPS‑induced COX‑2 expression and reduced COX‑2‑derived prostaglandin E2 and pro‑inflammatory cytokine secretion. Furthermore, a co‑culture system was used to reveal that overexpression of KLF9 in RAW 264.7 cells promoted HepG2 cell survival. In summary, it is reported that KLF9 promoted apoptosis of proinflammatory macrophages, and suppressed the antitumor effects, which can be selectively targeted by GCs as a novel mechanism to suppress antineoplastic activity.
Keywords: glucocorticoids; macrophages; dexamethasone; KLF9; apoptosis; immunosuppression; COX-2.
Figures
References
-
- Cari L, De Rosa F, Nocentini G, Riccardi C. Context-dependent effect of glucocorticoids on the proliferation, differentiation, and apoptosis of regulatory T cells: A review of the empirical evidence and clinical applications. Int J Mol Sci. 2019;20(pii):E1142. doi: 10.3390/ijms20051142. - DOI - PMC - PubMed
-
- Mylka V, Deckers J, Ratman D, De Cauwer L, Thommis J, De Rycke R, Impens F, Libert C, Tavernier J, Vanden Berghe W, et al. The autophagy receptor SQSTM1/p62 mediates anti-inflammatory actions of the selective NR3C1/glucocorticoid receptor modulator compound A (CpdA) in macrophages. Autophagy. 2018;14:2049–2064. doi: 10.1080/15548627.2018.1495681. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

