Protective effects of human umbilical cord blood‑derived mesenchymal stem cells against dexamethasone‑induced apoptotic cell death in hair follicles
- PMID: 31894311
- PMCID: PMC6984800
- DOI: 10.3892/ijmm.2019.4447
Protective effects of human umbilical cord blood‑derived mesenchymal stem cells against dexamethasone‑induced apoptotic cell death in hair follicles
Abstract
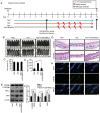
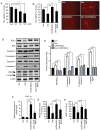
Alopecia is a common and distressing condition, and developing new therapeutic agents to prevent hair loss is important. Human umbilical cord blood‑derived mesenchymal stem cells (hUCB‑MSCs) have been studied intensively in regenerative medicine. However, the therapeutic potential of these cells against hair loss and hair organ damage remains unclear, and the effects of hUCB‑MSC transplantation on hair loss require evaluation. The current study aimed to investigate the effects of hUCB‑MSCs on hair regression in vivo and restoration of anagen conduction on hair growth in vitro. The effects of hUCB‑MSCs were explored in mouse catagen induction models using a topical treatment of 0.1% dexamethasone to induce hair regression. Dexamethasone was also used to simulate a stress environment in vitro. The results demonstrated that hUCB‑MSCs significantly prevented hair regression induced by dexamethasone topical stimulation in vivo. Additionally, hUCB‑MSCs significantly increased the proliferation of human dermal papilla cells (hDPCs) and HaCaT cells, which are key constituent cells of the hair follicle. Stimulation of vascular endothelial growth factor secretion and decreased expression of DKK‑1 by hUCB‑MSCs were also observed in hDPCs. Restoration of cell viability by hUCB‑MSCs suggested that these cells exerted a protective effect on glucocorticoid stress‑associated hair loss. In addition, anti‑apoptotic effects and regulation of the autophagic flux recovery were observed in HaCaT cells. The results of the present study indicated that hUCB‑MSCs may have the capacity to protect hair follicular dermal papilla cells and keratinocytes, thus preventing hair loss. Additionally, the protective effects of hUCB‑MSCs may be resistant to dysregulation of autophagy under harmful stress.
Keywords: hair follicle; hUcB-MScs; alopecia; stem-cell therapy; glucocorticoid.
Figures





References
-
- Lim JY, Jeong CH, Jun JA, Kim SM, Ryu CH, Hou Y, Oh W, Chang JW, Jeun SS. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia. Stem Cell Res Ther. 2011;2:38. doi: 10.1186/scrt79. - DOI - PMC - PubMed

