Diffusion magnetic resonance imaging-derived free water detects neurodegenerative pattern induced by interferon-γ
- PMID: 31894407
- PMCID: PMC7003714
- DOI: 10.1007/s00429-019-02017-1
Diffusion magnetic resonance imaging-derived free water detects neurodegenerative pattern induced by interferon-γ
Abstract
Imaging biomarkers for immune activation may be valuable for early-stage detection, therapeutic testing, and research on neurodegenerative conditions. In the present study, we determined whether diffusion magnetic resonance imaging-derived free water signal is a sensitive marker for neuroinflammatory effects of interferon-gamma (Ifn-γ). Neonatal wild-type mice were injected in the cerebral ventricles with recombinant adeno-associated viruses expressing the inflammatory cytokine Ifn-γ. Groups of mice expressing Ifn-γ and age-matched controls were imaged at 1, 5 and 8 months. Mice deficient in Ifngr1-/- and Stat1-/- were scanned at 5 months as controls for the signaling cascades activated by Ifn-γ. The results indicate that Ifn-γ affected fractional anisotropy (FA), mean diffusivity (MD), and free water (FW) in white matter structures, midline cortical areas, and medial thalamic areas. In these structures, FA and MD decreased progressively from 1 to 8 months of age, while FW increased significantly. The observed reductions in FA and MD and increased FW with elevated brain Ifn-γ was not observed in Ifngr1-/- or Stat1-/- mice. These results suggest that the observed microstructure changes involve the Ifn-gr1 and Stat1 signaling. Interestingly, increases in FW were observed in midbrain of Ifngr1-/- mice, which suggests alternative Ifn-γ signaling in midbrain. Although initial evidence is offered in relation to the sensitivity of the FW signal to neurodegenerative and/or inflammatory patterns specific to Ifn-γ, further research is needed to determine applicability and specificity across animal models of neuroinflammatory and degenerative disorders.
Keywords: Aging; Diffusion MRI; Free water; Inflammation; Interferon gamma; White matter.
Conflict of interest statement
Competing interests
Authors declare that they have no competing interests.
Figures

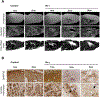

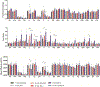


References
-
- Albi A, Pasternak O, Minati L, Marizzoni M, Bartres-Faz D, Bargallo N, Bosch B, Rossini PM, Marra C, Muller B, Fiedler U, Wiltfang J, Roccatagliata L, Picco A, Nobili FM, Blin O, Sein J, Ranjeva JP, Didic M, Bombois S, Lopes R, Bordet R, Gros-Dagnac H, Payoux P, Zoccatelli G, Alessandrini F, Beltramello A, Ferretti A, Caulo M, Aiello M, Cavaliere C, Soricelli A, Parnetti L, Tarducci R, Floridi P, Tsolaki M, Constantinidis M, Drevelegas A, Frisoni G, Jovicich J, PharmaCog C (2017) Free water elimination improves test-retest reproducibility of diffusion tensor imaging indices in the brain: A longitudinal multisite study of healthy elderly subjects. Hum Brain Mapp 38 (1):12–26. doi:10.1002/hbm.23350 - DOI - PMC - PubMed
-
- Ayers JI, Fromholt S, Sinyavskaya O, Siemienski Z, Rosario AM, Li A, Crosby KW, Cruz PE, DiNunno NM, Janus C, Ceballos-Diaz C, Borchelt DR, Golde TE, Chakrabarty P, Levites Y (2015) Widespread and efficient transduction of spinal cord and brain following neonatal AAV injection and potential disease modifying effect in ALS mice. Mol Ther 23 (1):53–62. doi:10.1038/mt.2014.180 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- Postdoctoral Fellowship/UF McKnight Brain Foundation
- T32 NS082168/NS/NINDS NIH HHS/United States
- P50NS091856/NS/NINDS NIH HHS/United States
- R01 NS052318/NS/NINDS NIH HHS/United States
- P50 AG047266/AG/NIA NIH HHS/United States
- S10 RR025671/RR/NCRR NIH HHS/United States
- P50AG047266/AG/NIA NIH HHS/United States
- R01 AG055798/AG/NIA NIH HHS/United States
- P30 AG066506/AG/NIA NIH HHS/United States
- 1R01AG055798/AG/NIA NIH HHS/United States
- R21 NS065273/NS/NINDS NIH HHS/United States
- R01NS052318/NS/NINDS NIH HHS/United States
- R01 NS075012/NS/NINDS NIH HHS/United States
- P50 NS091856/NS/NINDS NIH HHS/United States
- R01NS075012/NS/NINDS NIH HHS/United States
- T32NS082168/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

