Effect of Anti-IL5, Anti-IL5R, Anti-IL13 Therapy on Asthma Exacerbations: A Network Meta-analysis
- PMID: 31894410
- PMCID: PMC9136642
- DOI: 10.1007/s00408-019-00310-8
Effect of Anti-IL5, Anti-IL5R, Anti-IL13 Therapy on Asthma Exacerbations: A Network Meta-analysis
Abstract
Background: Several new treatments for severe asthma have become available in the last decade; yet, little data exist to guide their use in specific patient populations.
Objective: A network meta-analysis was conducted comparing the efficacy of FDA-approved monoclonal antibody therapies in preventing exacerbations in patients with severe eosinophilic asthma.
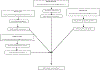
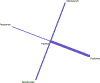
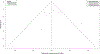
Methods: PubMed and Ovid were searched from inception until July 2019 for randomized controlled trials that studied the efficacy of benralizumab, dupilumab, mepolizumab, and reslizumab, in preventing acute exacerbations of asthma. Studies were included if they reported data for patients with severe eosinophilic asthma (defined in this meta-analysis as absolute eosinophil count ≥ 250 cells/μL). Annualized rate ratios for asthma exacerbations (during treatment) were calculated and converted to log rate ratios. Direct and indirect treatment estimates (for inter-drug differences) were analyzed using frequentist network meta-analysis methodology in R and treatments were ranked based on P-scores.
Results: In total, nine studies were included in the final analysis. Network meta-analysis revealed that all drugs were superior to placebo in preventing rates of asthma exacerbation in the study population and no inter-drug differences existed. Dupilumab was found to have the greatest magnitudes of effect on decreasing log rate ratio of asthma exacerbation based on P-score (0.83).
Conclusion: Benralizumab, dupilumab, mepolizumab, and reslizumab are all associated with decreased asthma exacerbations in patients with eosinophilic asthma, with no significant inter-drug differences.
Keywords: Benralizumab; Dupilumab; Eosinophilic asthma; Mepolizumab; Reslizumab.
Conflict of interest statement
Compliance with Ethical Standards
Figures
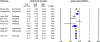
Similar articles
-
Comparative Efficacy of Anti IL-4, IL-5 and IL-13 Drugs for Treatment of Eosinophilic Asthma: A Network Meta-analysis.Lung. 2018 Oct;196(5):517-530. doi: 10.1007/s00408-018-0151-5. Epub 2018 Aug 23. Lung. 2018. PMID: 30167841
-
Reslizumab Compared with Benralizumab in Patients with Eosinophilic Asthma: A Systematic Literature Review and Network Meta-Analysis.J Allergy Clin Immunol Pract. 2019 Jan;7(1):122-130.e1. doi: 10.1016/j.jaip.2018.08.036. Epub 2018 Sep 11. J Allergy Clin Immunol Pract. 2019. PMID: 30217529
-
Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: Indirect treatment comparison.J Allergy Clin Immunol. 2019 Jan;143(1):190-200.e20. doi: 10.1016/j.jaci.2018.08.031. Epub 2018 Sep 8. J Allergy Clin Immunol. 2019. PMID: 30205189 Review.
-
Evidence for the efficacy and safety of anti-interleukin-5 treatment in the management of refractory eosinophilic asthma.Ther Adv Respir Dis. 2015 Aug;9(4):135-45. doi: 10.1177/1753465815581279. Epub 2015 Apr 21. Ther Adv Respir Dis. 2015. PMID: 25900924 Review.
-
Anti-IL-5 therapies for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2020 Dec 8;12(12):CD013432. doi: 10.1002/14651858.CD013432.pub2. Cochrane Database Syst Rev. 2020. PMID: 33295032 Free PMC article.
Cited by
-
Biologic Therapies for Severe Asthma: Current Insights and Future Directions.J Clin Med. 2025 May 2;14(9):3153. doi: 10.3390/jcm14093153. J Clin Med. 2025. PMID: 40364184 Free PMC article. Review.
-
Insight into IL-5 as a Potential Target for the Treatment of Allergic Diseases.Biomedicines. 2024 Jul 10;12(7):1531. doi: 10.3390/biomedicines12071531. Biomedicines. 2024. PMID: 39062104 Free PMC article. Review.
-
Comparative efficacy of biologics for patients with inadequately controlled asthma: A network meta-analysis.World Allergy Organ J. 2024 Jul 18;17(7):100934. doi: 10.1016/j.waojou.2024.100934. eCollection 2024 Jul. World Allergy Organ J. 2024. PMID: 39091592 Free PMC article.
-
Changes in Type 2 Biomarkers After Anti-IL5 Treatment in Patients With Severe Eosinophilic Asthma.Allergy Asthma Immunol Res. 2021 Mar;13(2):330-338. doi: 10.4168/aair.2021.13.2.330. Allergy Asthma Immunol Res. 2021. PMID: 33474865 Free PMC article.
-
LUNG Year in Review: 2020.Lung. 2021 Feb;199(1):1-5. doi: 10.1007/s00408-021-00418-w. Epub 2021 Jan 26. Lung. 2021. PMID: 33496843 Free PMC article. No abstract available.
References
-
- https://ginasthma.org/severeasthma/. Accessed 4 Aug. April 2019 edition
-
- Iftikhar IH, Schimmel M, Bender W, Swenson C, Amrol D (2018) Comparative efficacy of anti IL-4, IL-5 and IL-13 drugs for treatment of eosinophilic asthma: a network meta-analysis. Lung 196:517–530 - PubMed
-
- He LL, Zhang L, Jiang L, Xu F, Fei DS (2018) Efficacy and safety of anti-interleukin-5 therapy in patients with asthma: a pairwise and Bayesian network meta-analysis. Int Immunopharmacol 64:223–231 - PubMed
-
- Busse W, Chupp G, Nagase H, Albers FC, Doyle S, Shen Q, Bratton DJ, Gunsoy NB (2019) Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol 143:190–200.e120 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

