Self-Assembled Cagelike Receptor That Binds Biologically Relevant Dicarboxylic Acids via Proton-Coupled Anion Recognition
- PMID: 31895551
- PMCID: PMC8009274
- DOI: 10.1021/jacs.9b11566
Self-Assembled Cagelike Receptor That Binds Biologically Relevant Dicarboxylic Acids via Proton-Coupled Anion Recognition
Abstract
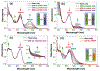
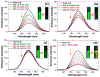
We report here a fully organic, self-assembled dimeric receptor, constructed from acyclic naphthyridyl-polypyrrolic building blocks. The cagelike dimer is stable in the solid state, in solution, and in gas phase, as inferred from X-ray diffraction and spectroscopic analyses. This system acts as a receptor for oxalic acid, maleic acid, and malonic acid in the solid state and in THF solution. In contrast, acetic acid, propionic acid, adipic acid, and succinic acid, with pKa values > ca. 2.8, were not bound effectively within the cagelike cavity. It is speculated that oxalic acid, maleic acid, and malonic acid serve to protonate the naphthyridine moieties of the host, which then favors binding of the corresponding carboxylate anions via hydrogen-bonding to the pyrrolic NH protons. The present naphthyridine-polypyrrole dimer is stable under acidic conditions, including in the presence of 100 equiv trifluoroacetic acid (TFA), p-toluenesulfonic acid (PTSA), H2SO4, and HCl. However, disassembly may be achieved by exposure to tetrabutylammonium fluoride (TBAF). Washing with water then regenerates the cage. This process of assembly and disassembly could be repeated >20 times with little evidence of degradation. The reversible nature of the present system, coupled with its dicarboxylic acid recognition features, leads us to suggest it could have a role to play in effecting the controlled "capture" and "release" of biologically relevant dicarboxylic acids.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
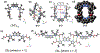




Similar articles
-
Solvent-Controlled Self-Assembled Oligopyrrolic Receptor.Molecules. 2021 Mar 22;26(6):1771. doi: 10.3390/molecules26061771. Molecules. 2021. PMID: 33809927 Free PMC article.
-
X-ray studies on crystalline complexes involving amino acids and peptides. XLII. Adipic acid complexes of L- and DL-arginine and supramolecular association in arginine-dicarboxylic acid complexes.Acta Crystallogr B. 2005 Feb;61(Pt 1):89-95. doi: 10.1107/S0108768104030010. Epub 2005 Jan 19. Acta Crystallogr B. 2005. PMID: 15659861
-
Cluster phase chemistry: gas-phase reactions of anionic sodium salts of dicarboxylic acid clusters with water molecules.J Phys Chem A. 2006 Jun 29;110(25):7777-86. doi: 10.1021/jp055944v. J Phys Chem A. 2006. PMID: 16789762
-
Coordination self-assembly of tetranuclear Pt(II) macrocycles with an organometallic backbone for sensing of acyclic dicarboxylic acids.Dalton Trans. 2013 Feb 28;42(8):2998-3008. doi: 10.1039/c2dt31828h. Dalton Trans. 2013. PMID: 23258385
-
Cluster phase chemistry: collisions of vibrationally excited cationic dicarboxylic acid clusters with water molecules initiate dissociation of cluster components.J Phys Chem A. 2007 Jul 12;111(27):5954-67. doi: 10.1021/jp0706580. Epub 2007 Jun 15. J Phys Chem A. 2007. PMID: 17569511
Cited by
-
Solution and Solid State Studies of Urea Derivatives of DITIPIRAM Acting as Powerful Anion Receptors.Molecules. 2021 Mar 22;26(6):1788. doi: 10.3390/molecules26061788. Molecules. 2021. PMID: 33810117 Free PMC article.
-
Open-Chain Tetrapyrroles Meet Metal Ions in the Functional Molecular Material Science.Chempluschem. 2025 Jun;90(6):e202500090. doi: 10.1002/cplu.202500090. Epub 2025 Apr 6. Chempluschem. 2025. PMID: 40126191 Free PMC article.
-
Solvent-Controlled Self-Assembled Oligopyrrolic Receptor.Molecules. 2021 Mar 22;26(6):1771. doi: 10.3390/molecules26061771. Molecules. 2021. PMID: 33809927 Free PMC article.
-
Visualizing molecular weights differences in supramolecular polymers.Proc Natl Acad Sci U S A. 2022 Mar 1;119(9):e2121746119. doi: 10.1073/pnas.2121746119. Proc Natl Acad Sci U S A. 2022. PMID: 35197296 Free PMC article.
References
-
- Fujita M; Tominaga M; Hori A; Therrien B Coordination Assemblies from a Pd(II)-Cornered Square Complex. Acc. Chem. Res 2005, 38, 369–378. - PubMed
-
- De S; Mahata K; Schmittel M Metal-coordination-drivendynamic heteroleptic architectures. Chem. Soc. Rev 2010, 39, 1555–1575. - PubMed
-
- Saha ML; Yan X; Stang PJ Photophysical Properties of Organoplatinum(II) Compounds and Derived Self-Assembled Metallacycles and Metallacages: Fluorescence and Its Applications. Acc. Chem. Res 2016, 49, 2527–2539. - PubMed
-
- Ueda Y; Ito H; Fujita D; Fujita M Permeable Self-Assembled Molecular Containers for Catalyst Isolation Enabling Two-Step Cascade Reactions. J. Am. Chem. Soc 2017, 139, 6090–6093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

