Enasidenib drives human erythroid differentiation independently of isocitrate dehydrogenase 2
- PMID: 31895700
- PMCID: PMC7108889
- DOI: 10.1172/JCI133344
Enasidenib drives human erythroid differentiation independently of isocitrate dehydrogenase 2
Abstract
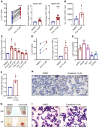
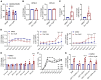
Cancer-related anemia is present in more than 60% of newly diagnosed cancer patients and is associated with substantial morbidity and high medical costs. Drugs that enhance erythropoiesis are urgently required to decrease transfusion rates and improve quality of life. Clinical studies have observed an unexpected improvement in hemoglobin and RBC transfusion-independence in patients with acute myeloid leukemia (AML) treated with the isocitrate dehydrogenase 2 (IDH2) mutant-specific inhibitor enasidenib, leading to improved quality of life without a reduction in AML disease burden. Here, we demonstrate that enasidenib enhanced human erythroid differentiation of hematopoietic progenitors. The phenomenon was not observed with other IDH1/2 inhibitors and occurred in IDH2-deficient CRISPR-engineered progenitors independently of D-2-hydroxyglutarate. The effect of enasidenib on hematopoietic progenitors was mediated by protoporphyrin accumulation, driving heme production and erythroid differentiation in committed CD71+ progenitors rather than hematopoietic stem cells. Our results position enasidenib as a promising therapeutic agent for improvement of anemia and provide the basis for a clinical trial using enasidenib to decrease transfusion dependence in a wide array of clinical contexts.
Keywords: Bone marrow differentiation; Hematology; Leukemias; Stem cells.
Conflict of interest statement
Figures


References
-
- [No authors listed]. SEER Cancer Stat Facts: Acute Myeloid Leukemia. National Cancer Institute. https://seer.cancer.gov/statfacts/html/amyl.html Accessed February 24, 2020.
-
- Yen K, et al. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 2017;7(5):478–493. doi: 10.1158/2159-8290.CD-16-1034. - DOI - PubMed
-
- Thomas D, Majeti R. Optimizing next-generation AML therapy: activity of mutant IDH2 inhibitor AG-221 in preclinical models. Cancer Discov. 2017;7(5):459–461. doi: 10.1158/2159-8290.CD-17-0270. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

