Applying the D50 disease progression model to gray and white matter pathology in amyotrophic lateral sclerosis
- PMID: 31896467
- PMCID: PMC6940701
- DOI: 10.1016/j.nicl.2019.102094
Applying the D50 disease progression model to gray and white matter pathology in amyotrophic lateral sclerosis
Abstract
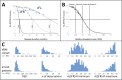
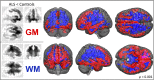
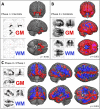
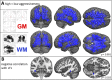
Therapeutic management and research in Amyotrophic Laterals Sclerosis (ALS) have been limited by the substantial heterogeneity in progression and anatomical spread that are endemic of the disease. Neuroimaging biomarkers represent powerful additions to the current monitoring repertoire but have yielded inconsistent associations with clinical scores like the ALS functional rating scale. The D50 disease progression model was developed to address limitations with clinical indices and the difficulty obtaining longitudinal data in ALS. It yields overall disease aggressiveness as time taken to reach halved functionality (D50); individual disease covered in distinct phases; and calculated functional state and calculated functional loss as acute descriptors of local disease activity. It greatly reduces the noise of the ALS functional rating scale and allows the comparison of highly heterogeneous disease and progression subtypes. In this study, we performed Voxel-Based Morphometry for 85 patients with ALS (60.1 ± 11.5 years, 36 female) and 62 healthy controls. Group-wise comparisons were performed separately for gray matter and white matter using ANCOVA testing with threshold-free cluster enhancement. ALS-related widespread gray and white matter density decreases were observed in the bilateral frontal and temporal lobes (p < 0.001, family-wise error corrected). We observed a progressive spread of structural alterations along the D50-derived phases, that were primarily located in frontal, temporal and occipital gray matter areas, as well as in supratentorial neuronal projections (p < 0.001 family-wise error corrected). ALS patients with higher overall disease aggressiveness (D50 < 30 months) showed a distinct pattern of supratentorial white matter density decreases relative to patients with lower aggressiveness; no significant differences were observed for gray matter density (p < 0.001 family-wise error corrected). The application of the D50 disease progression model separates measures of disease aggressiveness from disease accumulation. It revealed a strong correlation between disease phases and in-vivo measures of cerebral structural integrity. This study underscores the proposed corticofugal spread of cerebral pathology in ALS. We recommend application of the D50 model in studies linking clinical data with neuroimaging correlates.
Keywords: Amyotrophic lateral sclerosis; D50 model; Disease progression; Magnetic resonance imaging; Voxel-Based Morphometry.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no conflicts of interest to declare.
Figures
References
-
- Abdulla S., Machts J., Kaufmann J., Patrick K., Kollewe K., Dengler R., Heinze H.J., Petri S., Vielhaber S., Nestor P.J. Hippocampal degeneration in patients with amyotrophic lateral sclerosis. Neurobiol. Aging. 2014;35:2639–2645. - PubMed
-
- Abrahams S., Newton J., Niven E., Foley J., Bak T.H. Screening for cognition and behaviour changes in ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 2014;15:9–14. - PubMed
-
- Agosta F., Gorno-Tempini M.L., Pagani E., Sala S., Caputo D., Perini M., Bartolomei I., Fruguglietti M.E., Filippi M. Longitudinal assessment of grey matter contraction in amyotrophic lateral sclerosis: a tensor based morphometry study. Amyotroph. Lateral Scler. 2009;10:168–174. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

