AR ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-Mediated redox homeostasis
- PMID: 31896509
- PMCID: PMC6940696
- DOI: 10.1016/j.redox.2019.101413
AR ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-Mediated redox homeostasis
Abstract
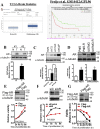
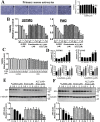
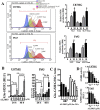
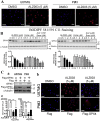
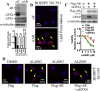
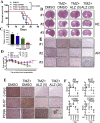
Drug resistance is the main obstacle in the improvement of chemotherapeutic efficacy in glioblastoma. Previously, we showed that dehydroepiandrosterone (DHEA), one kind of androgen/neurosteroid, potentiates glioblastoma to acquire resistance through attenuating DNA damage. Androgen receptor (AR) activated by DHEA or other types of androgen was reported to promote drug resistance in prostate cancer. However, in DHEA-enriched microenvironment, the role of AR in acquiring resistance of glioblastoma remains unknown. In this study, we found that AR expression is significantly correlated with poor prognosis, and AR obviously induced the resistance to temozolomide (TMZ) treatment. Herein, we observed that ALZ003, a curcumin analog, induces FBXL2-mediated AR ubiquitination, leading to degradation. Importantly, ALZ003 significantly inhibited the survival of TMZ-sensitive and -resistant glioblastoma in vitro and in vivo. The accumulation of reactive oxygen species (ROS), lipid peroxidation and suppression of glutathione peroxidase (GPX) 4, which are characteristics of ferroptosis, were observed in glioblastoma cell after treatment of ALZ003. Furthermore, overexpression of AR prevented ferroptosis in the presence of GPX4. To evaluate the therapeutic effect in vivo, we transplanted TMZ-sensitive or -resistant U87MG cells into mouse brain followed by intravenous administration with ALZ003. In addition to inhibiting the growth of glioblastoma, ALZ003 significantly extended the survival period of transplanted mice, and significantly decreased AR expression in the tumor area. Taken together, AR potentiates TMZ resistance for glioblastoma, and ALZ003-mediated AR ubiquitination might open a new insight into therapeutic strategy for TMZ resistant glioblastoma.
Keywords: ALZ003; AR; GPX4; Glioblastoma.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest TCC, CHY and HC are employed by Allianz Pharmascience Ltd. when this collaborative study was conducted.
Figures


References
-
- Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., Curschmann J., Janzer R.C., Ludwin S.K., Gorlia T., Allgeier A., Lacombe D., Cairncross J.G., Eisenhauer E., Mirimanoff R.O., European Organisation for R., Treatment of Cancer Brain T., Radiotherapy G., National Cancer Institute of Canada Clinical Trials G. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

