Clinical Features and Outcomes of Immune Checkpoint Inhibitor-Associated AKI: A Multicenter Study
- PMID: 31896554
- PMCID: PMC7003302
- DOI: 10.1681/ASN.2019070676
Clinical Features and Outcomes of Immune Checkpoint Inhibitor-Associated AKI: A Multicenter Study
Abstract
Background: Despite increasing recognition of the importance of immune checkpoint inhibitor-associated AKI, data on this complication of immunotherapy are sparse.
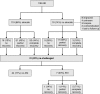
Methods: We conducted a multicenter study of 138 patients with immune checkpoint inhibitor-associated AKI, defined as a ≥2-fold increase in serum creatinine or new dialysis requirement directly attributed to an immune checkpoint inhibitor. We also collected data on 276 control patients who received these drugs but did not develop AKI.
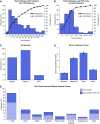
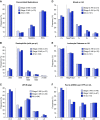
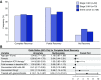
Results: Lower baseline eGFR, proton pump inhibitor use, and combination immune checkpoint inhibitor therapy were each independently associated with an increased risk of immune checkpoint inhibitor-associated AKI. Median (interquartile range) time from immune checkpoint inhibitor initiation to AKI was 14 (6-37) weeks. Most patients had subnephrotic proteinuria, and approximately half had pyuria. Extrarenal immune-related adverse events occurred in 43% of patients; 69% were concurrently receiving a potential tubulointerstitial nephritis-causing medication. Tubulointerstitial nephritis was the dominant lesion in 93% of the 60 patients biopsied. Most patients (86%) were treated with steroids. Complete, partial, or no kidney recovery occurred in 40%, 45%, and 15% of patients, respectively. Concomitant extrarenal immune-related adverse events were associated with worse renal prognosis, whereas concomitant tubulointerstitial nephritis-causing medications and treatment with steroids were each associated with improved renal prognosis. Failure to achieve kidney recovery after immune checkpoint inhibitor-associated AKI was independently associated with higher mortality. Immune checkpoint inhibitor rechallenge occurred in 22% of patients, of whom 23% developed recurrent associated AKI.
Conclusions: This multicenter study identifies insights into the risk factors, clinical features, histopathologic findings, and renal and overall outcomes in patients with immune checkpoint inhibitor-associated AKI.
Keywords: acute kidney injury; immune checkpoint inhibitors; tubulointerstitial nephritis.
Copyright © 2020 by the American Society of Nephrology.
Figures
References
-
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al.: KEYNOTE-189 Investigators : Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378: 2078–2092, 2018 - PubMed
-
- Wei SC, Duffy CR, Allison JP: Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8: 1069–1086, 2018 - PubMed
-
- Postow MA, Sidlow R, Hellmann MD: Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158–168, 2018 - PubMed
-
- Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al.: ESMO Guidelines Committee : Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28[Suppl 4]: iv119–iv142, 2017 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

