Generation, Coordination, and Evolution of Neural Circuits for Vocal Communication
- PMID: 31896561
- PMCID: PMC6939475
- DOI: 10.1523/JNEUROSCI.0736-19.2019
Generation, Coordination, and Evolution of Neural Circuits for Vocal Communication
Abstract
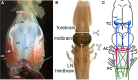
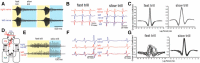
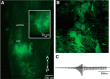
In many species, vocal communication is essential for coordinating social behaviors including courtship, mating, parenting, rivalry, and alarm signaling. Effective communication requires accurate production, detection, and classification of signals, as well as selection of socially appropriate responses. Understanding how signals are generated and how acoustic signals are perceived is key to understanding the neurobiology of social behaviors. Here we review our long-standing research program focused on Xenopus, a frog genus which has provided valuable insights into the mechanisms and evolution of vertebrate social behaviors. In Xenopus laevis, vocal signals differ between the sexes, through development, and across the genus, reflecting evolutionary divergence in sensory and motor circuits that can be interrogated mechanistically. Using two ex vivo preparations, the isolated brain and vocal organ, we have identified essential components of the vocal production system: the sexually differentiated larynx at the periphery, and the hindbrain vocal central pattern generator (CPG) centrally, that produce sex- and species-characteristic sound pulse frequencies and temporal patterns, respectively. Within the hindbrain, we have described how intrinsic membrane properties of neurons in the vocal CPG generate species-specific vocal patterns, how vocal nuclei are connected to generate vocal patterns, as well as the roles of neurotransmitters and neuromodulators in activating the circuit. For sensorimotor integration, we identified a key forebrain node that links auditory and vocal production circuits to match socially appropriate vocal responses to acoustic features of male and female calls. The availability of a well supported phylogeny as well as reference genomes from several species now support analysis of the genetic architecture and the evolutionary divergence of neural circuits for vocal communication. Xenopus thus provides a vertebrate model in which to study vocal communication at many levels, from physiology, to behavior, and from development to evolution. As one of the most comprehensively studied phylogenetic groups within vertebrate vocal communication systems, Xenopus provides insights that can inform social communication across phyla.
Keywords: CPG; duets; hindbrain; neuroendocrine; parabrachial; song.
Copyright © 2020 the authors.
Figures





References
-
- Albersheim-Carter J, Blubaum A, Ballagh IH, Missaghi K, Siuda ER, McMurray G, Bass AH, Dubuc R, Kelley DB, Schmidt MF, Wilson RJ, Gray PA (2016) Testing the evolutionary conservation of vocal motoneurons in vertebrates. Respir Physiol Neurobiol 224:2–10. 10.1016/j.resp.2015.06.010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
