Repurposing Papaverine as an Antiviral Agent against Influenza Viruses and Paramyxoviruses
- PMID: 31896588
- PMCID: PMC7158703
- DOI: 10.1128/JVI.01888-19
Repurposing Papaverine as an Antiviral Agent against Influenza Viruses and Paramyxoviruses
Abstract
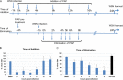
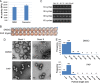
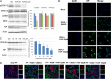
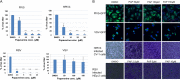
Influenza viruses are highly infectious and are the leading cause of human respiratory diseases and may trigger severe epidemics and occasional pandemics. Although antiviral drugs against influenza viruses have been developed, there is an urgent need to design new strategies to develop influenza virus inhibitors due to the increasing resistance of viruses toward currently available drugs. In this study, we examined the antiviral activity of natural compounds against the following influenza virus strains: A/WSN/33 (H1N1), A/Udorn/72 (H3N2), and B/Lee/40. Papaverine (a nonnarcotic alkaloid that has been used for the treatment of heart disease, impotency, and psychosis) was found to be an effective inhibitor of multiple strains of influenza virus. Kinetic studies demonstrated that papaverine inhibited influenza virus infection at a late stage in the virus life cycle. An alteration in influenza virus morphology and viral ribonucleoprotein (vRNP) localization was observed as an effect of papaverine treatment. Papaverine is a well-known phosphodiesterase inhibitor and also modifies the mitogen-activated protein kinase (MAPK) pathway by downregulating the phosphorylation of MEK and extracellular signal-regulated kinase (ERK). Thus, the modulation of host cell signaling pathways by papaverine may be associated with the nuclear retention of vRNPs and the reduction of influenza virus titers. Interestingly, papaverine also inhibited paramyxoviruses parainfluenza virus 5 (PIV5), human parainfluenza virus 3 (HPIV3), and respiratory syncytial virus (RSV) infections. We propose that papaverine can be a potential candidate to be used as an antiviral agent against a broad range of influenza viruses and paramyxoviruses.IMPORTANCE Influenza viruses are important human pathogens that are the causative agents of epidemics and pandemics. Despite the availability of an annual vaccine, a large number of cases occur every year globally. Here, we report that papaverine, a vasodilator, shows inhibitory action against various strains of influenza virus as well as the paramyxoviruses PIV5, HPIV3, and RSV. A significant effect of papaverine on the influenza virus morphology was observed. Papaverine treatment of influenza-virus-infected cells resulted in the inhibition of virus at a later time in the virus life cycle through the suppression of nuclear export of vRNP and also interfered with the host cellular cAMP and MEK/ERK cascade pathways. This study explores the use of papaverine as an effective inhibitor of both influenza viruses as well as paramyxoviruses.
Keywords: ERK; MAPK; MEK; cAMP; influenza virus; inhibitors; nuclear export; papaverine; paramyxovirus; phosphodiesterase; vRNP.
Copyright © 2020 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

