Rotavirus Reprograms Multiple Interferon Receptors and Restricts Their Intestinal Antiviral and Inflammatory Functions
- PMID: 31896593
- PMCID: PMC7158711
- DOI: 10.1128/JVI.01775-19
Rotavirus Reprograms Multiple Interferon Receptors and Restricts Their Intestinal Antiviral and Inflammatory Functions
Abstract
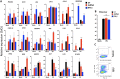
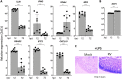
Rotaviruses (RV) cause acute severe diarrhea in the absence of substantial intestinal inflammation. They are also highly infectious in their homologous host species. The replication capacity of RV in the small bowel is substantially due to its ability to inhibit different types of interferons (IFNs). Here, we found that during RV infection in vitro, both virus-infected and uninfected bystander cells resist STAT1 phosphorylation and interferon regulatory factor 7 (IRF7) induction in response to exogenous interferon (IFN). Functionally, cellular transcription in response to stimulation with IFN, but not intracellular double-stranded RNA (dsRNA), was inhibited by RV. Further, IFNAR1 stimulation during RV infection significantly repressed a set of virus-induced transcripts. Regulation of IFN signaling in vivo was studied in suckling mice using the highly infectious murine EW RV strain. Kinetic studies indicated that sustained EW RV replication and IFN induction in the small intestine are accompanied by significant decreases in IFN-stimulated transcripts. Lipopolysaccharide (LPS)-mediated intestinal damage, driven by STAT1-induced inflammation, was also prevented in EW RV-infected mice. Remarkably, by ectopically stimulating either IFNAR1 or IFNGR1 in EW RV-infected mice, we could eliminate several intestinal antiviral and inflammatory transcriptional responses to RV. In contrast to infection with homologous RV, infection with a STAT1-sensitive heterologous RV strain induced IFN-stimulated transcripts, inflammatory cytokines, and intestinal expression of STAT1-pY701. Finally, RV strain-specific STAT1 regulation also likely determines the intestinal activation of multiple caspases. The simian RRV strain, but not murine EW RV, uniquely triggers the cleavage of both extrinsic and intrinsic caspases (caspases 8, 9, and 3) in a STAT1-mediated manner. Collectively, our findings reveal efficient reprograming of multiple IFN receptors toward a negative-feedback mode of signaling, accompanied by suppression of IFN-mediated antiviral, apoptotic, and inflammatory functions, during natural RV intestinal infection.IMPORTANCE Rotavirus is a highly infectious pathogen that causes severe diarrhea. Replication of RV in the small intestine is restricted to homologous host species, and host range restriction is substantially determined by the interferon response. In this study, we demonstrate that during infection, RV bystander cells resist exogenous IFN-mediated STAT1 signaling and transcription. In a suckling mouse model, ectopically stimulating different intestinal interferon receptors during RV infection eliminates several innate and inflammatory antiviral responses. Different intestinal inflammatory cytokines were also suppressed by homologous RV, as was intestinal damage in response to endotoxin. The ability of RV to suppress IFN-mediated receptors likely impacts intestinal cell homeostasis, as the cleavage of multiple intestinal caspases during RV infection is mediated by the IFN-STAT1 signaling pathway. Together, our results provide a mechanism underlying both the remarkable interferon resistance of homologous RV and its ability to prevent substantial inflammatory damage to the small bowel.
Keywords: STAT1; caspase cleavage; endotoxin; gut inflammation; interferon receptor; interferons; negative feedback; rotavirus.
Copyright © 2020 American Society for Microbiology.
Figures




References
-
- Lin JD, Feng N, Sen A, Balan M, Tseng HC, McElrath C, Smirnov SV, Peng J, Yasukawa LL, Durbin RK, Durbin JE, Greenberg HB, Kotenko SV. 2016. Distinct roles of type I and type III interferons in intestinal immunity to homologous and heterologous rotavirus infections. PLoS Pathog 12:e1005600. doi:10.1371/journal.ppat.1005600. - DOI - PMC - PubMed
-
- Sen A, Rothenberg ME, Mukherjee G, Feng N, Kalisky T, Nair N, Johnstone IM, Clarke MF, Greenberg HB. 2012. Innate immune response to homologous rotavirus infection in the small intestinal villous epithelium at single-cell resolution. Proc Natl Acad Sci U S A 109:20667–20672. doi:10.1073/pnas.1212188109. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

