Grafting Embryonic Raphe Neurons Reestablishes Serotonergic Regulation of Sympathetic Activity to Improve Cardiovascular Function after Spinal Cord Injury
- PMID: 31896670
- PMCID: PMC7002146
- DOI: 10.1523/JNEUROSCI.1654-19.2019
Grafting Embryonic Raphe Neurons Reestablishes Serotonergic Regulation of Sympathetic Activity to Improve Cardiovascular Function after Spinal Cord Injury
Abstract
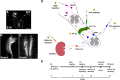
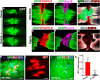
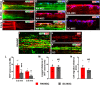
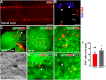
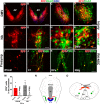
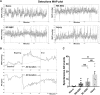
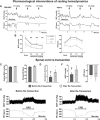
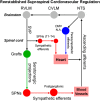
Cardiovascular dysfunction often occurs after high-level spinal cord injury. Disrupting supraspinal vasomotor pathways affects basal hemodynamics and contributes to the development of autonomic dysreflexia (AD). Transplantation of early-stage neurons to the injured cord may reconstruct the descending projections to enhance cardiovascular performance. To determine the specific role of reestablishing serotonergic regulation of hemodynamics, we implanted serotonergic (5-HT+) neuron-enriched embryonic raphe nucleus-derived neural stem cells/progenitors (RN-NSCs) into a complete spinal cord transection lesion site in adult female rats. Grafting embryonic spinal cord-derived NSCs or injury alone served as 2 controls. Ten weeks after injury/grafting, histological analysis revealed well-survived grafts and partial integration with host tissues in the lesion site. Numerous graft-derived serotonergic axons topographically projected to the caudal autonomic regions. Neuronal tracing showed that host supraspinal vasomotor pathways regenerated into the graft, and 5-HT+ neurons within graft and host brainstem neurons were transsynaptically labeled by injecting pseudorabies virus (PRV-614) into the kidney, indicating reconnected serotonergic circuits regulating autonomic activity. Using an implanted telemeter to record cardiovascular parameters, grafting RN-NSCs restored resting mean arterial pressure to normal levels and remarkably alleviated naturally occurring and colorectal distension-induced AD. Subsequent pharmacological blockade of 5-HT2A receptors with ketanserin in RN-NSC-grafted rats reduced resting mean arterial pressure and increased heart rate in all but 2 controls. Furthermore, spinal cord retransection below RN-NSC grafts partially eliminated the recovery in AD. Collectively, these data indicate that RN-NSCs grafted into a spinal cord injury site relay supraspinal control of serotonergic regulation for sympathetic activity to improve cardiovascular function.SIGNIFICANCE STATEMENT Disruption of supraspinal vasomotor pathways results in cardiovascular dysfunction following high-level spinal cord injury. To reestablish the descending regulation of autonomic function, we transplanted serotonergic neuron enriched embryonic raphe nucleus-derived neural stem cells/progenitors into the lesion site of completely transected rat spinal cord. Consequently, grafted raphe nucleus-derived neural stem cells/progenitors acted as a neuronal relay to reconnect supraspinal center and spinal sympathetic neurons below the injury. The reconstituted serotonergic regulation of sympathetic activity led to the improvement of hemodynamic parameters and mitigated autonomic dysreflexia. Based on morphological and physiological results, this study validates the effectiveness of transplanting early-stage serotonergic neurons into the spinal cord for cardiovascular functional recovery after spinal cord injury.
Keywords: cell transplantation; hemodynamics; spinal cord transection; transsynaptic tracing.
Copyright © 2020 the authors.
Figures




Similar articles
-
Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection.J Neurosci. 2013 Oct 23;33(43):17138-49. doi: 10.1523/JNEUROSCI.2851-13.2013. J Neurosci. 2013. PMID: 24155317 Free PMC article.
-
Development of Cardiovascular Dysfunction in a Rat Spinal Cord Crush Model and Responses to Serotonergic Interventions.J Neurotrauma. 2019 May 1;36(9):1478-1486. doi: 10.1089/neu.2018.5962. Epub 2019 Jan 8. J Neurotrauma. 2019. PMID: 30362884 Free PMC article.
-
Characterization of supraspinal vasomotor pathways and autonomic dysreflexia after spinal cord injury in F344 rats.Auton Neurosci. 2013 Jun;176(1-2):54-63. doi: 10.1016/j.autneu.2013.02.001. Epub 2013 Mar 7. Auton Neurosci. 2013. PMID: 23466042
-
Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
-
Autonomic Dysreflexia in Spinal Cord Injury: Mechanisms and Prospective Therapeutic Targets.Neuroscientist. 2024 Oct;30(5):597-611. doi: 10.1177/10738584231217455. Epub 2023 Dec 12. Neuroscientist. 2024. PMID: 38084412 Free PMC article. Review.
Cited by
-
The Effects of Extrinsic and Intrinsic Factors on Neurogenesis.Cells. 2023 Apr 29;12(9):1285. doi: 10.3390/cells12091285. Cells. 2023. PMID: 37174685 Free PMC article. Review.
-
NF-κB inhibition attenuates sympathetic hyperreflexia and concomitant development of autonomic dysreflexia and immune dysfunction after spinal cord injury.Commun Biol. 2025 May 22;8(1):787. doi: 10.1038/s42003-025-08237-y. Commun Biol. 2025. PMID: 40404889 Free PMC article.
-
Modern advances in spinal cord regeneration: hydrogel combined with neural stem cells.Front Pharmacol. 2024 Jun 27;15:1419797. doi: 10.3389/fphar.2024.1419797. eCollection 2024. Front Pharmacol. 2024. PMID: 38994202 Free PMC article. Review.
-
Cell transplantation to repair the injured spinal cord.Int Rev Neurobiol. 2022;166:79-158. doi: 10.1016/bs.irn.2022.09.008. Epub 2022 Nov 9. Int Rev Neurobiol. 2022. PMID: 36424097 Free PMC article. No abstract available.
-
Generation of a TPH2-EGFP reporter cell line for purification and monitoring of human serotonin neurons in vitro and in vivo.Stem Cell Reports. 2022 Oct 11;17(10):2365-2379. doi: 10.1016/j.stemcr.2022.08.012. Epub 2022 Sep 22. Stem Cell Reports. 2022. PMID: 36150384 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
