Memory engrams: Recalling the past and imagining the future
- PMID: 31896692
- PMCID: PMC7577560
- DOI: 10.1126/science.aaw4325
Memory engrams: Recalling the past and imagining the future
Abstract
In 1904, Richard Semon introduced the term "engram" to describe the neural substrate for storing memories. An experience, Semon proposed, activates a subset of cells that undergo off-line, persistent chemical and/or physical changes to become an engram. Subsequent reactivation of this engram induces memory retrieval. Although Semon's contributions were largely ignored in his lifetime, new technologies that allow researchers to image and manipulate the brain at the level of individual neurons has reinvigorated engram research. We review recent progress in studying engrams, including an evaluation of evidence for the existence of engrams, the importance of intrinsic excitability and synaptic plasticity in engrams, and the lifetime of an engram. Together, these findings are beginning to define an engram as the basic unit of memory.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Competing interests:
The authors declare no competing interests.
Figures

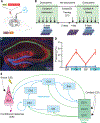


References
-
- Schacter DL, Stranger Behind the Engram: Theories of Memory and the Psychology of Science (Erlbaum Associates, 1982).
-
- Schacter DL, Eich JE, Tulving E, Richard Semon’s theory of memory. J. Verbal Learn. Verbal Behav 17, 721–743 (1978). doi: 10.1016/S0022-5371(78)90443-7 - DOI
-
- Semon R, The Mneme (G. Allen & Unwin, 1921).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

