Tissue clearing and its applications in neuroscience
- PMID: 31896771
- PMCID: PMC8121164
- DOI: 10.1038/s41583-019-0250-1
Tissue clearing and its applications in neuroscience
Erratum in
-
Publisher Correction: Tissue clearing and its applications in neuroscience.Nat Rev Neurosci. 2020 May;21(5):298. doi: 10.1038/s41583-020-0291-5. Nat Rev Neurosci. 2020. PMID: 32152524
Abstract
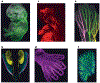
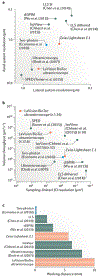
State-of-the-art tissue-clearing methods provide subcellular-level optical access to intact tissues from individual organs and even to some entire mammals. When combined with light-sheet microscopy and automated approaches to image analysis, existing tissue-clearing methods can speed up and may reduce the cost of conventional histology by several orders of magnitude. In addition, tissue-clearing chemistry allows whole-organ antibody labelling, which can be applied even to thick human tissues. By combining the most powerful labelling, clearing, imaging and data-analysis tools, scientists are extracting structural and functional cellular and subcellular information on complex mammalian bodies and large human specimens at an accelerated pace. The rapid generation of terabyte-scale imaging data furthermore creates a high demand for efficient computational approaches that tackle challenges in large-scale data analysis and management. In this Review, we discuss how tissue-clearing methods could provide an unbiased, system-level view of mammalian bodies and human specimens and discuss future opportunities for the use of these methods in human neuroscience.
Conflict of interest statement
Competing interests
H.R.U. is a co-inventor on a patent applications covering the CUBIC reagents (PCT/JP2014/070618 (pending), patent applicant is RIKEN, other co-inventors are E. A. Susaki and K. Tainaka; PCT/JP2017/016410 (pending), patent applicant is RIKEN, other co-inventors are K. Tainaka and T. Murakami) and a co-founder of CUBICStars Inc. A.E. is the applicant and the inventor on a patent application for technologies relating to vDISCO clearing (PCT/EP2018/063098 (pending)). K.C. is the inventor or a co-inventor on patents and patent applications for CLARITY (PCT/US2013/031066 (active), patent applicant is Stanford University, co-inventor is K. A. Deisseroth), stochastic electrotransport (PCT/US2015/024297 (active), patent applicant is MIT), SHIELD (PCT/US2016/064538 (pending), applicant is Massachusetts Institute of Technology (MIT), other co-inventors are E. Murray and J. H. Cho), SWITCH (PCT/US2016/064538 (pending), applicant is MIT, other co-inventors are E. Murray and J. H. Cho) and MAP (PCT/US2017/030285 (pending), applicant is MIT, other co-inventors are T. Ku, J. M. Swaney and J. Y. Park) and a co-founder of LifeCanvas Technologies. V.G. is a co-inventor on patent applications covering PACT and PARS (PCT/US2014/048985 (active), applicant is California Institute of Technology, other co-inventors are V. Gradinaru and B. Yang) and adeno-associated virus (US14/485,024 (active), applicant is California Institute of Technology, other co-inventors are B. E. Deverman, P. H. Patterson and V. Gradinaru) technologies. P.J.K. is an inventor or co-inventor on patents and patent applications covering multiview imaging (US14/049,470 (active), applicant is Howard Hughes Medical Institute) and adaptive light-sheet microscopy (PCT/US2017/038970 (pending), applicant is Howard Hughes Medical Institute, other co-inventors are R. K. Chhetri and L. A. Royer). P.T. and A.C. declare no competing interests.
Figures



References
-
- Spalteholz W Über das Durchsichtigmachen von Menschlichen und Tierischen Präparaten. (S. Hirzel, 1914).
-
- Yang B et al. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 158, 945–958 (2014). - PMC - PubMed
-
This article first reports clearing of adult rodents via the vasculature; RNA detection in cleared tissues with single-molecule resolution by single-molecule fluorescence in situ hybridization and that specific formulations of tissue–hydrogel hybrids can significantly expand.
-
- Renier N et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014. - PubMed
-
- Susaki EA et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157, 726–739 (2014. - PubMed
-
This article first reports on the delipidation activity of amino alcohols and the high-performance hydrophilic tissue-clearing method CUBIC and reports tissue clearing of the marmoset brain.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

