A retrospective observational study to estimate the attrition of patients across lines of systemic treatment for metastatic colorectal cancer in Canada
- PMID: 31896945
- PMCID: PMC6927777
- DOI: 10.3747/co.26.4861
A retrospective observational study to estimate the attrition of patients across lines of systemic treatment for metastatic colorectal cancer in Canada
Abstract
Background: Selection and sequencing of treatment regimens for individual patients with metastatic colorectal cancer (mcrc) is driven by maintaining reasonable quality of life and extending survival, as well as by access to and cost of therapies. The objectives of the present study were to describe, for patients with mcrc, attrition across lines of systemic therapy, patterns of therapy and their timing, and KRAS status.
Methods: A retrospective chart review at 6 Canadian academic centres included sequential patients who were diagnosed with mcrc from 1 January 2009 onward and who initiated first-line systemic treatment for mcrc between 1 January and 31 December 2009. Death was included as a competing risk in the analysis.
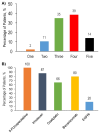
Results: The analysis included 200 patients who started first-line therapy. The proportions of patients who started second-, third-, and fourth-line systemic therapy were 70%, 30%, and 15% respectively. Chemotherapy plus bevacizumab was the most common first-line combination (66%). The most common first-line regimen was folfiri plus bevacizumab. KRAS testing was performed in 103 patients (52%), and 38 of 68 patients (56%, 19% overall) with confirmed KRAS wild-type tumours received an epidermal growth factor receptor inhibitor (egfri), which was more common in later lines. Most KRAS testing occurred after initiation of second-line therapy.
Conclusions: In the modern treatment era, a high proportion of patients receive at least two lines of therapy for mcrc, but only 19% receive egfri therapy. Earlier KRAS testing and therapy with an egfri might allow a greater proportion of patients to access all 5 active treatment agents.
Keywords: KRAS testing; Treatment patterns; anti-vascular growth factor agents; chemotherapy; epidermal growth factor inhibitor.
2019 Multimed Inc.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: This study was funded by Amgen. HK has participated in clinical trials and advisory boards for Hoffman–La Roche, Sanofi, and Amgen. SB has participated in advisory boards sponsored by Amgen and Lilly, and has received speaking honoraria from Amgen. JM has participated in clinical trials, advisory boards, and educational events for Amgen. PK has received educational grants from Amgen, Novartis, Ipsen Biopharmaceuticals, Celgene, and Taiho Pharma Canada, and has participated in advisory boards with Amgen, Novartis, Ipsen Biopharmaceuticals, Celgene, Taiho Pharma Canada, Pfizer, and AstraZeneca. NA has participated in clinical trials and advisory boards with Amgen and Roche. FC has no conflicts of interest to disclose. MPC and BG are former employees of Amgen Canada and have stock or stock options in the company.
Figures
References
-
- National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. Ver. 2.2019. Fort Washington, PA: NCCN; 2019. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (free registration required); cited 16 May 2019]
-
- Bristol–Myers Squibb Canada. Bristol–Myers Squibb Canada commercializes Erbitux for advanced colorectal cancer [online news release] Montreal, QC: Bristol–Myers Squibb Canada; 2008. [Available at: https://www.bms.com/ca/en/media/press-release-listing/2008-10-19-press-r...; cited 26 May 2019]
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

