Adverse drug reactions in drug information databases: does presentation affect interpretation?
- PMID: 31897054
- PMCID: PMC6919994
- DOI: 10.5195/jmla.2020.748
Adverse drug reactions in drug information databases: does presentation affect interpretation?
Abstract
Objective: Formatting of adverse drug reaction (ADR) information differs among drug information (DI) resources and may impact clinical decision-making. The objective of this study was to determine whether ADR formatting impacts adverse event interpretation by pharmacy practitioners and students.
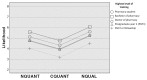
Methods: Participants were randomized to receive ADR information in a comparative quantitative (CQUANT), noncomparative quantitative (NQUANT), or noncomparative qualitative (NQUAL) format to interpret 3 clinical vignettes. Vignettes involved patients presenting with adverse events that varied in the extent to which they were associated with a medication. The primary outcome was interpretation of the likelihood of medication-induced adverse events on a 10-point Likert scale. Lower scoring on likelihood (i.e., ADR deemed unlikely) reflected more appropriate interpretation. Linear regression was performed to analyze the effects of ADR information format on the primary outcome.
Results: A total of 108 participants completed the study (39 students and 69 pharmacists). Overall, the CQUANT group had the lowest average likelihood compared to NQUAL (4.0 versus 5.4; p<0.01) and NQUANT (4.0 versus 4.9; p=0.016) groups. There was no difference between NQUAL and NQUANT groups (5.4 versus 4.9; p=0.14). In the final model, at least 2 years of postgraduate training (-1.1; 95% CI: -1.8 to -0.3; p<0.01) and CQUANT formatting (-1.3; 95% CI: -0.9 to -1.7; p<0.01) were associated with reduced likelihood.
Conclusions: Formatting impacts pharmacists' and pharmacy students' interpretation of ADR information. CQUANT formatting and at least two years of postgraduate training improved interpretation of adverse events.
Copyright: © 2020, Authors.
Figures
Similar articles
-
Evaluation of adverse drug reaction formatting in drug information databases.J Med Libr Assoc. 2020 Oct 1;108(4):598-604. doi: 10.5195/jmla.2020.983. J Med Libr Assoc. 2020. PMID: 33013217 Free PMC article.
-
Evaluation of adverse drug reaction formatting in drug information mobile phone applications.J Med Libr Assoc. 2022 Jan 1;110(1):81-86. doi: 10.5195/jmla.2022.1251. J Med Libr Assoc. 2022. PMID: 35210966 Free PMC article.
-
Factors that influence under-reporting of suspected adverse drug reactions among community pharmacists in a Spanish region.Drug Saf. 2007;30(11):1073-82. doi: 10.2165/00002018-200730110-00006. Drug Saf. 2007. PMID: 17973543
-
Pilot testing of checklists to discern adverse drug reactions and adverse drug events.J Am Pharm Assoc (2003). 2013 Jan-Feb;53(1):61-9. doi: 10.1331/JAPhA.2013.11196. J Am Pharm Assoc (2003). 2013. PMID: 23636158 Review.
-
Pharmacovigilance: pharmacists' perspective on spontaneous adverse drug reaction reporting.Integr Pharm Res Pract. 2017 Mar 22;6:91-98. doi: 10.2147/IPRP.S105881. eCollection 2017. Integr Pharm Res Pract. 2017. PMID: 29354555 Free PMC article. Review.
Cited by
-
Evaluation of adverse drug reaction formatting in drug information databases.J Med Libr Assoc. 2020 Oct 1;108(4):598-604. doi: 10.5195/jmla.2020.983. J Med Libr Assoc. 2020. PMID: 33013217 Free PMC article.
-
Evaluation of adverse drug reaction formatting in drug information mobile phone applications.J Med Libr Assoc. 2022 Jan 1;110(1):81-86. doi: 10.5195/jmla.2022.1251. J Med Libr Assoc. 2022. PMID: 35210966 Free PMC article.
References
-
- Institute for Safe Medication Practices. Baxter and the Institute for Safe Medication Practices (ISMP) address global medication error prevention [Internet] The Institute; 2017. [cited 21 May 2019]. < https://www.ismp.org/news/baxter-and-institute-safe-medication-practices...>.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials