Clinical relevance of neutralizing antibodies in botulinum toxin long-term treated still-responding patients with cervical dystonia
- PMID: 31897089
- PMCID: PMC6918489
- DOI: 10.1177/1756286419892078
Clinical relevance of neutralizing antibodies in botulinum toxin long-term treated still-responding patients with cervical dystonia
Abstract
Background: The aim of the study was to test the clinical relevance of neutralizing antibodies (NABs) in patients with cervical dystonia (CD) still responding to repeat injections with botulinum toxin type A (BoNT/A).
Methods: Enzyme-linked immunosorbent assay (ELISA)-test evidence from a cross-sectional study on 221 CD-patients with treatment durations of between 2 and 21 years and still responding to repeat BoNT/A-injections showed the presence of antibodies against BoNT/A in 39 patients. A mouse hemi-diaphragm (MHDA) confirmation test was performed in these 39 ELISA-positive patients, and demographic (age, sex, age at onset of CD) and treatment-related (duration of treatment, mean dose of the last 10 injections, TSUI-score, patient's subjective scoring of the treatment effect, patient's scoring of quality of life by means of the CDQ24-questionnaire) data from these 39 patients were compared with data from ELISA-negative patients. Paralysis time, the MHDA outcome measure, was correlated with clinical data.
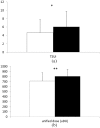
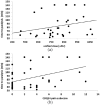
Results: The ELISA-positive CD-patients had significantly higher TSUI-scores (p < 0.015), and had been treated for significant longer (p < 0.022) and with significantly higher doses (p < 0.001). Patient's rating of BoNT/A-treatment effect and quality of life tended to be worse in ELISA-positive compared with ELISA-negative patients. The paralysis time of ELISA-positive patients was significantly correlated with the mean dose of the last 10 injections (p < 0.027) and the pain subscore of the CDQ24 (p < 0.012).
Conclusions: Presence of NABs is clinically relevant in CD, leading to a significantly worse head position, therapy with significantly higher BoNT/A doses, and a correlation between the CDQ24 pain-subscore and antibody titers.
Keywords: cervical dystonia; clinical relevance; long-term botulinum toxin treatment; neutralizing antibodies; secondary treatment failure.
© The Author(s), 2019.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
References
-
- Greene P, Fahn S, Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord 1994; 9: 213–217. - PubMed
LinkOut - more resources
Full Text Sources

