Direct single-molecule quantification reveals unexpectedly high mechanical stability of vinculin-talin/α-catenin linkages
- PMID: 31897422
- PMCID: PMC6920023
- DOI: 10.1126/sciadv.aav2720
Direct single-molecule quantification reveals unexpectedly high mechanical stability of vinculin-talin/α-catenin linkages
Abstract
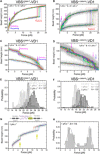
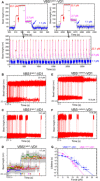
The vinculin-mediated mechanosensing requires establishment of stable mechanical linkages between vinculin to integrin at focal adhesions and to cadherins at adherens junctions through associations with the respective adaptor proteins talin and α-catenin. However, the mechanical stability of these critical vinculin linkages has yet to be determined. Here, we developed a single-molecule detector assay to provide direct quantification of the mechanical lifetime of vinculin association with the vinculin binding sites in both talin and α-catenin, which reveals a surprisingly high mechanical stability of the vinculin-talin and vinculin-α-catenin interfaces that have a lifetime of >1000 s at forces up to 10 pN and can last for seconds to tens of seconds at 15 to 25 pN. Our results suggest that these force-bearing intermolecular interfaces provide sufficient mechanical stability to support the vinculin-mediated mechanotransduction at cell-matrix and cell-cell adhesions.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
-
- Vogel V., Sheetz M., Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 7, 265–275 (2006). - PubMed
-
- Borgon R. A., Vonrhein C., Bricogne G., Bois P. R. J., Izard T., Crystal structure of human vinculin. Structure 12, 1189–1197 (2004). - PubMed
-
- Xu W., Baribault H., Adamson E. D., Vinculin knockout results in heart and brain defects during embryonic development. Development 125, 327–337 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

