Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial
- PMID: 31897524
- PMCID: PMC6997246
- DOI: 10.1007/s00125-019-05065-8
Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial
Abstract
Aims/hypothesis: Intra-abdominal or visceral obesity is associated with insulin resistance and an increased risk for cardiovascular disease. This study aimed to compare the effects of semaglutide 1.0 mg and canagliflozin 300 mg on body composition in a subset of participants from the SUSTAIN 8 Phase IIIB, randomised double-blind trial who underwent whole-body dual-energy x-ray absorptiometry (DXA) scanning.
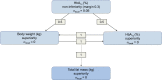
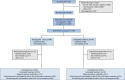
Methods: Adults (age ≥18 years) with type 2 diabetes, HbA1c 53-91 mmol/mol (7.0-10.5%), on a stable daily dose of metformin (≥1500 mg or maximum tolerated dose) and with an eGFR ≥60 ml min-1 [1.73 m]-2 were randomised 1:1 to semaglutide 1.0 mg once weekly and canagliflozin placebo once daily, or canagliflozin 300 mg once daily and semaglutide placebo once weekly. Body composition was assessed using whole-body DXA scans. The study participants and investigator remained blinded throughout the trial, and quality of DXA scans was evaluated in a blinded manner. Change from baseline to week 52 in total fat mass (kg) was the confirmatory efficacy endpoint.
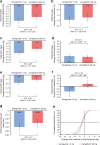
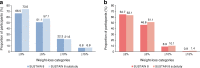
Results: A subset of 178 participants (semaglutide, n = 88; canagliflozin, n = 90) underwent DXA scanning at screening and were randomised into the substudy. Of these, 114 (semaglutide, n = 53; canagliflozin, n = 61) participants had observed end-of-treatment data included in the confirmatory efficacy analysis. Of the 178 participants in the substudy, numerical improvements in body composition (including fat mass, lean mass and visceral fat mass) were observed after 52 weeks with both treatments. Total fat mass (baseline 33.2 kg) was reduced by 3.4 kg and 2.6 kg with semaglutide and canagliflozin, respectively (estimated treatment difference: -0.79 [95% CI -2.10, 0.51]). Although total lean mass (baseline 51.3 kg) was also reduced by 2.3 kg and 1.5 kg with semaglutide and canagliflozin, respectively (estimated treatment difference: -0.78 [-1.61, 0.04]), the proportion of lean mass (baseline 59.4%) increased by 1.2%- and 1.1%-point, respectively (estimated treatment difference 0.14 [-0.89, 1.17]). Changes in visceral fat mass and overall changes in body composition (assessed by the fat to lean mass ratio) were comparable between the two treatment groups.
Conclusions/interpretation: In individuals with uncontrolled type 2 diabetes on stable-dose metformin therapy, the changes in body composition with semaglutide and canagliflozin were not significantly different. Although numerical improvements in body composition were observed following treatment in both treatment arms, the specific impact of both treatments on body composition in the absence of a placebo arm is speculative at this stage.
Trial registration: ClinicalTrials.gov NCT03136484.
Funding: This trial was supported by Novo Nordisk A/S, Denmark.
Keywords: Body composition; Canagliflozin; Fat mass; Glucagon-like peptide receptor agonists; Randomised controlled trial; Semaglutide; Type 2 diabetes; Weight.
Figures

References
-
- Gatineau M, Hancock C, Holman N et al (2014) Adult obesity and type 2 diabetes. Available from https://assets.publishing.service.gov.uk/government/uploads/system/uploa.... Accessed 25 November 2019
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

