Potential oncogenic roles of mutant-p53-derived exosomes in the tumor-host interaction of head and neck cancers
- PMID: 31897662
- PMCID: PMC11027799
- DOI: 10.1007/s00262-019-02450-5
Potential oncogenic roles of mutant-p53-derived exosomes in the tumor-host interaction of head and neck cancers
Abstract
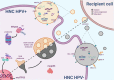
The wide-ranging collection of malignancies arising at the upper aerodigestive tract is categorized as head and neck cancer (HNC), the sixth most prevalent cancer worldwide. Infection with human papillomavirus (HPV) or exposure to carcinogens is the leading causes of HPV+ and HPV- HNCs development, respectively. HPV+ and HPV- HNCs are different in clinical and molecular aspects. Specifically, HPV- HNCs tightly associate with missense mutants of the TP53 gene (encoding for the p53 protein), suggesting a central role for mutant p53 gain-of-function (GOF) in driving tumorigenesis. In contrast, in HPV + HNC, the sequence of TP53 typically remains intact, while the protein is degraded. In tumor cells, the status of the TP53 gene affects the cargo of secreted exosomes. In this review, we describe the accumulated knowledge regarding the involvement of exosomes and p53 on cellular interactions between HPV+ and HPV- HNC cells, and the surrounding tumor microenvironment (TME). Moreover, we envision how TP53 status may determine exosomes cargo in HNC, and, consequently, modify the TME. The potential roles of exosomes described herein are based on both our studies and the studies of others on mutant p53-derived exosomes. Specifically, we showed how exosomes are shed by cancer cells harboring mutant p53 communicate with tumor-associated macrophages in the colon as well as with cancer-associated fibroblasts in the lung, creating immunosuppressive conditions and promoting invasiveness. Altogether, exosomes in HNC in the context of TP53 status are understudied and extensive research is required to shed light on the biology of HPV+ and HPV- HNC.
Keywords: CITIM 2019; Exosomes; Head and neck; Human papillomavirus; Mutant p53; Tumor microenvironment.
Conflict of interest statement
The authors declare that no potential conflicts of interest exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

