A mouse model for benign paroxysmal positional vertigo with genetic predisposition for displaced otoconia
- PMID: 31898392
- PMCID: PMC7286769
- DOI: 10.1111/gbb.12635
A mouse model for benign paroxysmal positional vertigo with genetic predisposition for displaced otoconia
Abstract
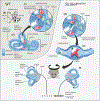
Abnormal formation of otoconia, the biominerals of the inner ear, results in balance disorders. The inertial mass of otoconia activates the underlying mechanosensory hair cells in response to change in head position primarily during linear and rotational acceleration. Otoconia associate exclusively with the two gravity receptors, the utricle and saccule. The cristae sensory epithelium is associated with an extracellular gelatinous matrix known as cupula, equivalent to otoconia. During head rotation, the inertia of endolymphatic fluids within the semicircular canals deflects the cupula of the corresponding crista and activates the underlying mechanosensory hair cells. It is believed that detached free-floating otoconia particles travel ectopically to the semicircular canal and cristae and are the culprit for benign paroxysmal positional vertigo (BPPV). The Slc26a4 mouse mutant harbors a missense mutation in pendrin. This mutation leads to impaired transport activity of pendrin and to defects in otoconia composition and distribution. All Slc26a4 loop/loop homozygous mutant mice are profoundly deaf but show inconsistent vestibular deficiency. A panel of behavioral tests was utilized in order to generate a scoring method for vestibular function. A pathological finding of displaced otoconia was identified consistently in the inner ears of mutant mice with severe vestibular dysfunction. In this work, we present a mouse model with a genetic predisposition for ectopic otoconia with a clinical correlation to BPPV. This unique mouse model can serve as a platform for further investigation of BPPV pathophysiology, and for developing novel treatment approaches in a live animal model.
Keywords: SLC26A4; balance; deafness; hearing; vertigo.
© 2020 John Wiley & Sons Ltd and International Behavioural and Neural Genetics Society.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare no conflict of interests in the current study.
Figures



References
-
- Nedzelski JM, Barber HO, McIlmoyl L. Diagnoses in a dizziness unit. J Otolaryngol. 1986;15:101–104. - PubMed
-
- Epley JM. New dimensions of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 1980;88:599–605. - PubMed
-
- Semont A, Freyss G, Vitte E. Curing the BPPV with a Liberatory Maneuver In: Clinical Testing of the Vestibular System. Karger Publishers; 1988. p. 290–3. - PubMed
-
- Brandt T, Daroff RB. Physical therapy for benign paroxysmal positional vertigo. Arch Otolaryngol - Head Neck Surg. 1980;106:484–485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

