The pathogen Moniliophthora perniciosa promotes differential proteomic modulation of cacao genotypes with contrasting resistance to witches´ broom disease
- PMID: 31898482
- PMCID: PMC6941324
- DOI: 10.1186/s12870-019-2170-7
The pathogen Moniliophthora perniciosa promotes differential proteomic modulation of cacao genotypes with contrasting resistance to witches´ broom disease
Abstract
Background: Witches' broom disease (WBD) of cacao (Theobroma cacao L.), caused by Moniliophthora perniciosa, is the most important limiting factor for the cacao production in Brazil. Hence, the development of cacao genotypes with durable resistance is the key challenge for control the disease. Proteomic methods are often used to study the interactions between hosts and pathogens, therefore helping classical plant breeding projects on the development of resistant genotypes. The present study compared the proteomic alterations between two cacao genotypes standard for WBD resistance and susceptibility, in response to M. perniciosa infection at 72 h and 45 days post-inoculation; respectively the very early stages of the biotrophic and necrotrophic stages of the cacao x M. perniciosa interaction.
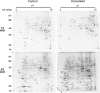
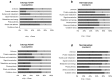
Results: A total of 554 proteins were identified, being 246 in the susceptible Catongo and 308 in the resistant TSH1188 genotypes. The identified proteins were involved mainly in metabolism, energy, defense and oxidative stress. The resistant genotype showed more expressed proteins with more variability associated with stress and defense, while the susceptible genotype exhibited more repressed proteins. Among these proteins, stand out pathogenesis related proteins (PRs), oxidative stress regulation related proteins, and trypsin inhibitors. Interaction networks were predicted, and a complex protein-protein interaction was observed. Some proteins showed a high number of interactions, suggesting that those proteins may function as cross-talkers between these biological functions.
Conclusions: We present the first study reporting the proteomic alterations of resistant and susceptible genotypes in the T. cacao x M. perniciosa pathosystem. The important altered proteins identified in the present study are related to key biologic functions in resistance, such as oxidative stress, especially in the resistant genotype TSH1188, that showed a strong mechanism of detoxification. Also, the positive regulation of defense and stress proteins were more evident in this genotype. Proteins with significant roles against fungal plant pathogens, such as chitinases, trypsin inhibitors and PR 5 were also identified, and they may be good resistance markers. Finally, important biological functions, such as stress and defense, photosynthesis, oxidative stress and carbohydrate metabolism were differentially impacted with M. perniciosa infection in each genotype.
Keywords: Disease resistance; Plant-pathogen interaction; Proteomics; Theobroma cacao.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Gramacho ICP, Mandarino EP, Matos AS. Cultivo e beneficiamento do cacau na Bahia. Ilhéus: CEPLAC; 1992.
-
- Aime MC, Phillips-Mora W. The causal agents of witches’ broom and frosty pod rot of cacao (chocolate, Theobroma cacao) form a new lineage of Marasmiaceae. Mycologia. 2005;97(5):1012–1022. - PubMed
-
- Pereira JL, Ram A, Figueredo JM, Almeida LCC. Primeira ocorrência de vassoura-de-bruxa na principal região produtora de cacau do Brasil. Agrotrópica. 1989;1(1):79–81.
-
- Evans HC. Pleomorphism in Crinipellis perniciosa, causal agent of Witches' broom disease of cocoa. Trans Br Mycol Soc. 1980;74(3):515–523. doi: 10.1016/S0007-1536(80)80051-9. - DOI
-
- Sena K, Alemanno L, Gramacho KP. The infection process of Moniliophthora perniciosa in cacao. Plant Pathol. 2014;63(3):1272–1281. doi: 10.1111/ppa.12224. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

