Drug-resistant enteric fever worldwide, 1990 to 2018: a systematic review and meta-analysis
- PMID: 31898501
- PMCID: PMC6941399
- DOI: 10.1186/s12916-019-1443-1
Drug-resistant enteric fever worldwide, 1990 to 2018: a systematic review and meta-analysis
Abstract
Background: Antimicrobial resistance (AMR) is an increasing threat to global health. There are > 14 million cases of enteric fever every year and > 135,000 deaths. The disease is primarily controlled by antimicrobial treatment, but this is becoming increasingly difficult due to AMR. Our objectives were to assess the prevalence and geographic distribution of AMR in Salmonella enterica serovars Typhi and Paratyphi A infections globally, to evaluate the extent of the problem, and to facilitate the creation of geospatial maps of AMR prevalence to help targeted public health intervention.
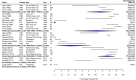
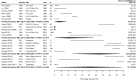
Methods: We performed a systematic review of the literature by searching seven databases for studies published between 1990 and 2018. We recategorised isolates to allow the analysis of fluoroquinolone resistance trends over the study period. The prevalence of multidrug resistance (MDR) and fluoroquinolone non-susceptibility (FQNS) in individual studies was illustrated by forest plots, and a random effects meta-analysis was performed, stratified by Global Burden of Disease (GBD) region and 5-year time period. Heterogeneity was assessed using the I2 statistics. We present a descriptive analysis of ceftriaxone and azithromycin resistance.
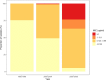
Findings: We identified 4557 articles, of which 384, comprising 124,347 isolates (94,616 S. Typhi and 29,731 S. Paratyphi A) met the pre-specified inclusion criteria. The majority (276/384; 72%) of studies were from South Asia; 40 (10%) articles were identified from Sub-Saharan Africa. With the exception of MDR S. Typhi in South Asia, which declined between 1990 and 2018, and MDR S. Paratyphi A, which remained at low levels, resistance trends worsened for all antimicrobials in all regions. We identified several data gaps in Africa and the Middle East. Incomplete reporting of antimicrobial susceptibility testing (AST) and lack of quality assurance were identified.
Interpretation: Drug-resistant enteric fever is widespread in low- and middle-income countries, and the situation is worsening. It is essential that public health and clinical measures, which include improvements in water quality and sanitation, the deployment of S. Typhi vaccination, and an informed choice of treatment are implemented. However, there is no licenced vaccine for S. Paratyphi A. The standardised reporting of AST data and rollout of external quality control assessment are urgently needed to facilitate evidence-based policy and practice.
Trial registration: PROSPERO CRD42018029432.
Keywords: Antimicrobial drug resistance; Azithromycin resistance; Ceftriaxone resistance; Drug-resistant infections; ESBL-producing; Enteric fever; Fluoroquinolone resistance; Harmonisation of breakpoints; Meta-analysis; Multidrug resistance; Paratyphoid fever; Prevalence of resistance; Salmonella Paratyphi A; Salmonella Typhi; Typhoid fever.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

