Addressing heterogeneity in amyotrophic lateral sclerosis CLINICAL TRIALS
- PMID: 31899540
- PMCID: PMC7496557
- DOI: 10.1002/mus.26801
Addressing heterogeneity in amyotrophic lateral sclerosis CLINICAL TRIALS
Abstract
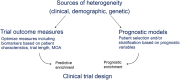
Amyotrophic lateral sclerosis (ALS) is a debilitating neurodegenerative disorder with complex biology and significant clinical heterogeneity. Many preclinical and early phase ALS clinical trials have yielded promising results that could not be replicated in larger phase 3 confirmatory trials. One reason for the lack of reproducibility may be ALS biological and clinical heterogeneity. Therefore, in this review, we explore sources of ALS heterogeneity that may reduce statistical power to evaluate efficacy in ALS trials. We also review efforts to manage clinical heterogeneity, including use of validated disease outcome measures, predictive biomarkers of disease progression, and individual clinical risk stratification. We propose that personalized prognostic models with use of predictive biomarkers may identify patients with ALS for whom a specific therapeutic strategy may be expected to be more successful. Finally, the rapid application of emerging clinical and biomarker strategies may reduce heterogeneity, increase trial efficiency, and, in turn, accelerate ALS drug development.
Keywords: amyotrophic lateral sclerosis; biomarkers; clinical trials; disease heterogeneity; enrichment strategies; outcome measures.
© 2020 The Authors. Muscle & Nerve published by Wiley Periodicals, Inc.
Conflict of interest statement
Namita Goyal has received research support from Amylyx Therapeutics, Brainstorm Cell Therapeutics, Cytokinetics, Orphazyme, and Orion and provided advisory board support for Cytokinetics, Biogen, Acceleron, and MT Pharma. James Berry has been a consultant for Clene Nanomedicine, Orion Pharmaceuticals, and Denali Therapeutics and has received research support from Anelixis Therapeutics, Amylyx Therapeutics, Brainstorm Cell Therapeutics, Biogen, Cytokinetics, MT Pharma of America, and Neuraltus Pharmaceuticals. Anthony Windebank has received support for conduct of clinical trials from BrainStorm Cell Therapeutics. Nathan Staff serves as site investigator for clinical trials funded by Brainstorm Cell Therapeutics and Orion Pharmaceuticals. Nicholas Maragakis serves on scientific advisory boards for Brainstorm Cell Therapeutics, Clene Nanomedicine and Orion Pharma. Leonard H. van den Berg serves on scientific advisory boards for the Biogen, Cytokinetics, Orion, and Sarepta. Angela Genge serves on the advisory boards of Avexis, Alexion, AL‐S Pharma, Biogen, Brainstorm, Akcea, Cytokinetics, Sanofi, Mitsubishi, and Novartis. Robert Miller has received research support from BrainStorm Cell Therapeutics, Cytokinetics, Amylyx, Neuraltus, Bioelectron and provided advisory board support for Cytokinetics, AveXis, Neuraltus and MT Pharma. Merit Cudkowicz has been a consultant for Biohaven, Biogen, Takeda, Avexis, and Revalesio and chaired DSMB for Lilly. Ralph Kern, Yael Gothelf and Chaim Lebovits are Brainstorm Cell Therapeutics employees. Robert Baloh reports no conflict of interest.
Figures

References
-
- Westeneng HJ, Debray TPA, Visser AE, et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol. 2018;17:423‐433. - PubMed
-
- Goyal NA, Cudkowicz ME, Berry JD, et al. A systematic review of enrichment strategies for current clinical trials. In: Proceedings from the ALS 29th International Symposium on ALS/MND; December 7‐9, 2018; Glasgow. United Kingdom..
-
- Maniatis S, Aijo T, Vickovic S, et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science. 2019;364:89‐93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

