Molecular mechanisms of vancomycin resistance
- PMID: 31899563
- PMCID: PMC7020976
- DOI: 10.1002/pro.3819
Molecular mechanisms of vancomycin resistance
Abstract
Vancomycin and related glycopeptides are drugs of last resort for the treatment of severe infections caused by Gram-positive bacteria such as Enterococcus species, Staphylococcus aureus, and Clostridium difficile. Vancomycin was long considered immune to resistance due to its bactericidal activity based on binding to the bacterial cell envelope rather than to a protein target as is the case for most antibiotics. However, two types of complex resistance mechanisms, each comprised of a multi-enzyme pathway, emerged and are now widely disseminated in pathogenic species, thus threatening the clinical efficiency of vancomycin. Vancomycin forms an intricate network of hydrogen bonds with the d-Ala-d-Ala region of Lipid II, interfering with the peptidoglycan layer maturation process. Resistance to vancomycin involves degradation of this natural precursor and its replacement with d-Ala-d-lac or d-Ala-d-Ser alternatives to which vancomycin has low affinity. Through extensive research over 30 years after the initial discovery of vancomycin resistance, remarkable progress has been made in molecular understanding of the enzymatic cascades responsible. Progress has been driven by structural studies of the key components of the resistance mechanisms which provided important molecular understanding such as, for example, the ability of this cascade to discriminate between vancomycin sensitive and resistant peptidoglycan precursors. Important structural insights have been also made into the molecular evolution of vancomycin resistance enzymes. Altogether this molecular data can accelerate inhibitor discovery and optimization efforts to reverse vancomycin resistance. Here, we overview our current understanding of this complex resistance mechanism with a focus on the structural and molecular aspects.
Keywords: antibiotic resistance; enzymes; glycopeptides; microbiology; structural biology; vancomycin.
© 2020 The Protein Society.
Figures



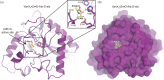

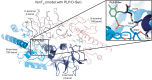

References
-
- CDC (2013) Antibiotic Resistance Threats in the United States, 2013.
-
- World Health Organization (WHO) . Antimicrobial resistance: global report on surveillance; 2014.
-
- Levine DP. Vancomycin: A history. Clin Infect Dis. 2006;42:S5–S12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

