In vivo elongation of thin filaments results in heart failure
- PMID: 31899774
- PMCID: PMC6941805
- DOI: 10.1371/journal.pone.0226138
In vivo elongation of thin filaments results in heart failure
Abstract
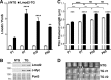
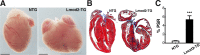
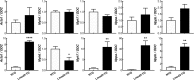
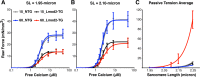
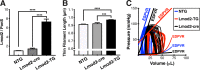
A novel cardiac-specific transgenic mouse model was generated to identify the physiological consequences of elongated thin filaments during post-natal development in the heart. Remarkably, increasing the expression levels in vivo of just one sarcomeric protein, Lmod2, results in ~10% longer thin filaments (up to 26% longer in some individual sarcomeres) that produce up to 50% less contractile force. Increasing the levels of Lmod2 in vivo (Lmod2-TG) also allows us to probe the contribution of Lmod2 in the progression of cardiac myopathy because Lmod2-TG mice present with a unique cardiomyopathy involving enlarged atrial and ventricular lumens, increased heart mass, disorganized myofibrils and eventually, heart failure. Turning off of Lmod2 transgene expression at postnatal day 3 successfully prevents thin filament elongation, as well as gross morphological and functional disease progression. We show here that Lmod2 has an essential role in regulating cardiac contractile force and function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

