Prospective evaluation of prognostic impact of KIT mutations on acute myeloid leukemia with RUNX1-RUNX1T1 and CBFB-MYH11
- PMID: 31899799
- PMCID: PMC6960455
- DOI: 10.1182/bloodadvances.2019000709
Prospective evaluation of prognostic impact of KIT mutations on acute myeloid leukemia with RUNX1-RUNX1T1 and CBFB-MYH11
Abstract
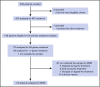
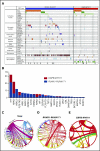
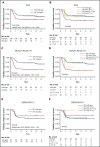
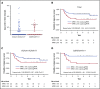
The prognostic impact of KIT mutation on core-binding factor acute myeloid leukemia (CBF-AML) remains controversial. We registered 199 newly diagnosed de novo CBF-AML patients, aged 16 to 64 years, who achieved complete remission. They received 3 courses of high-dose cytarabine therapy and no further treatment until hematological relapse. Mutations in exons 8, 10-11, and 17 of the KIT gene were analyzed. Furthermore, we analyzed mutations in 56 genes that are frequently identified in myeloid malignancies and evaluated minimal residual disease (MRD). The primary end point was relapse-free survival (RFS) according to KIT mutations. The RFS in KIT-mutated patients was inferior to that in unmutated patients (hazard ratio, 1.92; 95% confidence interval, 1.23-3.00; P = .003). Based on subgroup analysis, KIT mutations had a prognostic impact in patients with RUNX1-RUNX1T1, but not in those with CBFB-MYH11, and only exon 17 mutation had a significant prognostic impact. Multivariate Cox regression analysis with stepwise selection revealed that the KIT exon 17 mutation and the presence of extramedullary tumors in patients with RUNX1-RUNX1T1, and loss of chromosome X or Y and NRAS mutation in patients with CBFB-MYH11 were poor prognostic factors for RFS. MRD was evaluated in 112 patients, and it was associated with a poorer RFS in the patients with CBFB-MYH11, but not in those with RUNX1-RUNX1T1. These results suggested that it is necessary to separately evaluate AML with RUNX1-RUNX1T1 or CBFB-MYH11 according to appropriate prognostic factors. This study was registered at www.umin.ac.jp/ctr/ as #UMIN000003434.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of interest disclosure: H. Kiyoi received research funding from Chugai Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Zenyaku Kogyo Co. Ltd., FUJIFILM Corporation, Astellas Pharma Inc., Otsuka Pharmaceutical Co. Ltd., Nippon Shinyaku Co. Ltd., Eisai Co. Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co. Ltd., Novartis Pharma K.K., Sumitomo Dainippon Pharma Co. Ltd., Sanofi K.K., and Celgene Corporation; consulting fees from Astellas Pharma Inc., Amgen Astellas BioPharma K.K., and Daiichi Sankyo Co. Ltd.; honoraria from Bristol-Myers Squibb, Astellas Pharma Inc., and Novartis Pharma K.K. N. Dobashi received research funding from Pfizer Japan Inc., Chugai Pharmaceutical Co. Ltd., Astellas Pharma Inc., Kyowa Hakko Kirin Co. Ltd., Zenyaku Kogyo Co. Ltd., Eisai Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Celgene Co., and Sysmex Co. N. Asou received research funding from Chugai Pharmaceutical Co. Ltd. and Toyama Chemical Co., Ltd., and consulting fees from SRL Inc. The remaining authors declare to competing financial interests.
Figures

References
-
- Marcucci G, Mrózek K, Ruppert AS, et al. . Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): a Cancer and Leukemia Group B study. J Clin Oncol. 2005;23(24):5705-5717. - PubMed
-
- Paschka P, Du J, Schlenk RF, et al. . Secondary genetic lesions in acute myeloid leukemia with inv(16) or t(16;16): a study of the German-Austrian AML Study Group (AMLSG). Blood. 2013;121(1):170-177. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

