Short-term resistance exercise inhibits neuroinflammation and attenuates neuropathological changes in 3xTg Alzheimer's disease mice
- PMID: 31900170
- PMCID: PMC6942350
- DOI: 10.1186/s12974-019-1653-7
Short-term resistance exercise inhibits neuroinflammation and attenuates neuropathological changes in 3xTg Alzheimer's disease mice
Abstract
Background: Both human and animal studies have shown beneficial effects of physical exercise on brain health but most tend to be based on aerobic rather than resistance type regimes. Resistance exercise has the advantage of improving both muscular and cardiovascular function, both of which can benefit the frail and the elderly. However, the neuroprotective effects of resistance training in cognitive impairment are not well characterized.
Methods: We evaluated whether short-term resistant training could improve cognitive function and pathological changes in mice with pre-existing cognitive impairment. Nine-month-old 3xTg mouse underwent a resistance training protocol of climbing up a 1-m ladder with a progressively heavier weight loading.
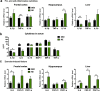
Results: Compared with sedentary counterparts, resistance training improved cognitive performance and reduced neuropathological and neuroinflammatory changes in the frontal cortex and hippocampus of mice. In line with these results, inhibition of pro-inflammatory intracellular pathways was also demonstrated.
Conclusions: Short-term resistance training improved cognitive function in 3xTg mice, and conferred beneficial effects on neuroinflammation, amyloid and tau pathology, as well as synaptic plasticity. Resistance training may represent an alternative exercise strategy for delaying disease progression in Alzheimer's disease.
Keywords: Alzheimer’s disease; Amyloid; Cytokines; Neuroinflammation; Resistance exercise; Synapse; Tau.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

