Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes
- PMID: 31900292
- PMCID: PMC7456731
- DOI: 10.1136/gutjnl-2019-319127
Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes
Abstract
Objective: High-fat diet (HFD)-induced metabolic disorders can lead to impaired sperm production. We aim to investigate if HFD-induced gut microbiota dysbiosis can functionally influence spermatogenesis and sperm motility.
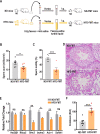
Design: Faecal microbes derived from the HFD-fed or normal diet (ND)-fed male mice were transplanted to the mice maintained on ND. The gut microbes, sperm count and motility were analysed. Human faecal/semen/blood samples were collected to assess microbiota, sperm quality and endotoxin.
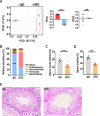
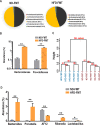
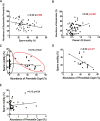
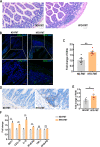
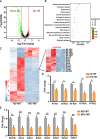
Results: Transplantation of the HFD gut microbes into the ND-maintained (HFD-FMT) mice resulted in a significant decrease in spermatogenesis and sperm motility, whereas similar transplantation with the microbes from the ND-fed mice failed to do so. Analysis of the microbiota showed a profound increase in genus Bacteroides and Prevotella, both of which likely contributed to the metabolic endotoxaemia in the HFD-FMT mice. Interestingly, the gut microbes from clinical subjects revealed a strong negative correlation between the abundance of Bacteroides-Prevotella and sperm motility, and a positive correlation between blood endotoxin and Bacteroides abundance. Transplantation with HFD microbes also led to intestinal infiltration of T cells and macrophages as well as a significant increase of pro-inflammatory cytokines in the epididymis, suggesting that epididymal inflammation have likely contributed to the impairment of sperm motility. RNA-sequencing revealed significant reduction in the expression of those genes involved in gamete meiosis and testicular mitochondrial functions in the HFD-FMT mice.
Conclusion: We revealed an intimate linkage between HFD-induced microbiota dysbiosis and defect in spermatogenesis with elevated endotoxin, dysregulation of testicular gene expression and localised epididymal inflammation as the potential causes.
Trial registration number: NCT03634644.
Keywords: diet; endotoxin; inflammation; intestinal microbiology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
Small intestinal bacterial overgrowth (SIBO) as a potential cause of impaired spermatogenesis.Gut. 2020 Nov;69(11):2058-2059. doi: 10.1136/gutjnl-2020-320766. Epub 2020 Feb 17. Gut. 2020. PMID: 32066624 Free PMC article. No abstract available.
-
Effects of high-fat diet-induced gut microbiota dysbiosis: far beyond the gut.Gut. 2020 Dec;69(12):2259. doi: 10.1136/gutjnl-2020-320717. Epub 2020 Feb 28. Gut. 2020. PMID: 32111631 No abstract available.
-
Reply to the comment.Gut. 2020 Dec;69(12):2259-2260. doi: 10.1136/gutjnl-2020-321220. Epub 2020 Apr 10. Gut. 2020. PMID: 32276951 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
