The effector mechanism of siRNA spherical nucleic acids
- PMID: 31900365
- PMCID: PMC6983385
- DOI: 10.1073/pnas.1915907117
The effector mechanism of siRNA spherical nucleic acids
Abstract
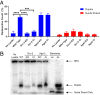
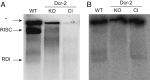
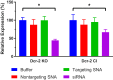
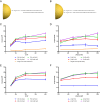
Spherical nucleic acids (SNAs) are nanostructures formed by chemically conjugating short linear strands of oligonucleotides to a nanoparticle template. When made with modified small interfering RNA (siRNA) duplexes, SNAs act as single-entity transfection and gene silencing agents and have been used as lead therapeutic constructs in several disease models. However, the manner in which modified siRNA duplex strands that comprise the SNA lead to gene silencing is not understood. Herein, a systematic analysis of siRNA biochemistry involving SNAs shows that Dicer cleaves the modified siRNA duplex from the surface of the nanoparticle, and the liberated siRNA subsequently functions in a way that is dependent on the canonical RNA interference mechanism. By leveraging this understanding, a class of SNAs was chemically designed which increases the siRNA content by an order of magnitude through covalent attachment of each strand of the duplex. As a consequence of increased nucleic acid content, this nanostructure architecture exhibits less cell cytotoxicity than conventional SNAs without a decrease in siRNA activity.
Keywords: gene regulation; siRNA processing; spherical nucleic acids.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Russ A. P., Lampel S., The druggable genome: An update. Drug Discov. Today 10, 1607–1610 (2005). - PubMed
-
- Conde J., Artzi N., Are RNAi and miRNA therapeutics truly dead? Trends Biotechnol. 33, 141–144 (2015). - PubMed
-
- Mirkin C. A., Letsinger R. L., Mucic R. C., Storhoff J. J., A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382, 607–609 (1996). - PubMed
-
- Rosi N. L., et al. , Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 312, 1027–1030 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

