Recommendations by a UK expert panel on an aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration
- PMID: 31900438
- PMCID: PMC7608090
- DOI: 10.1038/s41433-019-0747-x
Recommendations by a UK expert panel on an aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration
Abstract
Objectives: This report aims to provide clear recommendations and practical guidance from a panel of UK retinal experts on an aflibercept treat-and-extend (T&E) pathway that can be implemented in clinical practice. These recommendations may help service providers across the NHS intending to implement a T&E approach, with the aim of effectively addressing the capacity and resource issues putting strain on UK neovascular age-related macular degeneration (nAMD) services while promoting patients' best interests throughout.
Methods: Two structured roundtable meetings of retinal specialists were held in London, UK on 7 December 2018 and 1 March 2019. These meetings were organised and funded by Bayer.
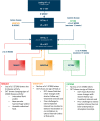
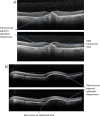
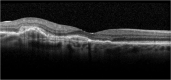
Results: The panel provided recommendations for an aflibercept T&E pathway and developed specific criteria based on visual acuity, retinal morphology and optical coherence tomography imaging to guide reduction, maintenance and extension of injection intervals. They also discussed the extension of treatment intervals by 2- or 4-week adjustments to a maximum treatment interval of 16 weeks, the management of retinal fluid and the stopping of treatment.
Conclusions: The long-term benefits of implementing a T&E pathway may include superior visual outcomes compared with a pro re nata (PRN; as needed) protocol, and a lower treatment burden compared with a fixed protocol, which is likely to improve service capacity. Furthermore, the predictable nature of a T&E approach compared with a PRN service may aid capacity planning for the future nAMD treatment demand.
Conflict of interest statement
All authors except NJ and NP received honoraria from Bayer plc UK. AR: Consulting fees from Bayer and Allergan; lecture fees from Bayer and Allergan. LD: Consulting and lecture fees from Bayer, Novartis, Alimera Sciences, Bausch + Lomb; grant support (travel) from Bayer, Novartis and Allergan; research funding from Bayer, Novartis, Allergan, Alimera Sciences and Roche. HD: Consulting fees from Bayer; lecture fees from Bayer; grant support (travel) from Bayer. RG: Consulting fees from Bayer and Novartis; research funding and educational grants from Bayer and Novartis. SM: Consulting fees from Bayer and Novartis; lecture fees from Bayer and Novartis; grant support (travel) from Bayer and Novartis; research funding from Roche. HM: Consulting fees from Bayer, Novartis, Roche and Allergan; research funding from Bayer, Novartis, Roche and Allergan. NN: Consulting fees from Bayer and Novartis; lecture fees from Bayer and Novartis; grant support (travel) from Bayer and Novartis. PJP: Consulting fees from Bayer and Novartis; research funding and educational grants from Bayer. NP: Owns Bayer shares and is employed by Bayer plc UK serving as a Medical Science Liaison. NJ: Owns Bayer shares and is employed by Bayer plc UK serving as a Senior Medical Advisor and MSL manager (Specialty Medicine – Ophthalmology).
Figures

References
-
- The Royal College of Ophthalmologists. The way forward: Age-related macular degeneration and diabetic retinopathy. https://www.rcophth.ac.uk/wpcontent/uploads/2015/10/RCOphth-The-Way-Forw.... Accessed Dec 2019.
-
- Lanzetta P, Loewenstein A, Vision Academy Steering Committee. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255:1259–73. doi: 10.1007/s00417-017-3647-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

