Harnessing big 'omics' data and AI for drug discovery in hepatocellular carcinoma
- PMID: 31900465
- PMCID: PMC7401304
- DOI: 10.1038/s41575-019-0240-9
Harnessing big 'omics' data and AI for drug discovery in hepatocellular carcinoma
Erratum in
-
Publisher Correction: Harnessing big 'omics' data and AI for drug discovery in hepatocellular carcinoma.Nat Rev Gastroenterol Hepatol. 2020 Mar 11:10.1038/s41575-020-0288-6. doi: 10.1038/s41575-020-0288-6. Online ahead of print. Nat Rev Gastroenterol Hepatol. 2020. PMID: 32161375 Free PMC article. No abstract available.
Abstract
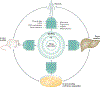
Hepatocellular carcinoma (HCC) is the most common form of primary adult liver cancer. After nearly a decade with sorafenib as the only approved treatment, multiple new agents have demonstrated efficacy in clinical trials, including the targeted therapies regorafenib, lenvatinib and cabozantinib, the anti-angiogenic antibody ramucirumab, and the immune checkpoint inhibitors nivolumab and pembrolizumab. Although these agents offer new promise to patients with HCC, the optimal choice and sequence of therapies remains unknown and without established biomarkers, and many patients do not respond to treatment. The advances and the decreasing costs of molecular measurement technologies enable profiling of HCC molecular features (such as genome, transcriptome, proteome and metabolome) at different levels, including bulk tissues, animal models and single cells. The release of such data sets to the public enhances the ability to search for information from these legacy studies and provides the opportunity to leverage them to understand HCC mechanisms, rationally develop new therapeutics and identify candidate biomarkers of treatment response. Here, we provide a comprehensive review of public data sets related to HCC and discuss how emerging artificial intelligence methods can be applied to identify new targets and drugs as well as to guide therapeutic choices for improved HCC treatment.
Conflict of interest statement
Competing interests
R.K.K. declares the following competing interests: research funding and/or supply of study drug to institution for conduct of clinical trials from Adaptimmune, Agios, AstraZeneca, Bayer, Bristol–Myers Squibb, Eli Lilly and Co, EMD Serono, Exelixis, Merck, Novartis, Partner Therapeutics, QED, Taiho; funding (to individual) for Independent Data Monitoring Committee membership by Genentech/Roche; Steering Committee/Advisory Board memberships (funding to institution) by Agios, AstraZeneca, Bristol–Myers Squibb; Steering Committee (without compensation): Exelixis. The other authors declare no competing interests.
Figures

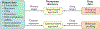
References
-
- Bray F et al. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 68, 394–424 (2018). - PubMed
-
- American Cancer Society. Key statistics about liver cancer. American Cancer Society https://www.cancer.org/cancer/liver-cancer/about/what-is-key-statistics.... (2019).

