Phase 1 Human Immunodeficiency Virus (HIV) Vaccine Trial to Evaluate the Safety and Immunogenicity of HIV Subtype C DNA and MF59-Adjuvanted Subtype C Envelope Protein
- PMID: 31900486
- PMCID: PMC7823071
- DOI: 10.1093/cid/ciz1239
Phase 1 Human Immunodeficiency Virus (HIV) Vaccine Trial to Evaluate the Safety and Immunogenicity of HIV Subtype C DNA and MF59-Adjuvanted Subtype C Envelope Protein
Abstract
Background: The Pox-Protein Public-Private Partnership is performing a suite of trials to evaluate the bivalent subtype C envelope protein (TV1.C and 1086.C glycoprotein 120) vaccine in the context of different adjuvants and priming agents for human immunodeficiency virus (HIV) type 1 (HIV-1) prevention.
Methods: In the HIV Vaccine Trials Network 111 trial, we compared the safety and immunogenicity of DNA prime followed by DNA/protein boost with DNA/protein coadministration injected intramuscularly via either needle/syringe or a needle-free injection device (Biojector). One hundred thirty-two healthy, HIV-1-uninfected adults were enrolled from Zambia, South Africa, and Tanzania and were randomized to 1 of 6 arms: DNA prime, protein boost by needle/syringe; DNA and protein coadministration by needle/syringe; placebo by needle/syringe; DNA prime, protein boost with DNA given by Biojector; DNA and protein coadministration with DNA given by Biojector; and placebo by Biojector.
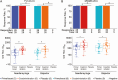
Results: All vaccinations were safe and well tolerated. DNA and protein coadministration was associated with increased HIV-1 V1/V2 antibody response rate, a known correlate of decreased HIV-1 infection risk. DNA administration by Biojector elicited significantly higher CD4+ T-cell response rates to HIV envelope protein than administration by needle/syringe in the prime/boost regimen (85.7% vs 55.6%; P = .02), but not in the coadministration regimen (43.3% vs 48.3%; P = .61).
Conclusions: Both the prime/boost and coadministration regimens are safe and may be promising for advancement into efficacy trials depending on whether cellular or humoral responses are desired.
Clinical trials registration: South African National Clinical Trials Registry (application 3947; Department of Health [DoH] no. DOH-27-0715-4917) and ClinicalTrials.gov (NCT02997969).
Keywords: Biojector; DNA prime/protein boost; HIV vaccine; subtype C.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures




References
-
- UNAIDS. Global AIDS update. 2018. Available at: http://www.unaids.org/sites/default/files/media_asset/miles-to-go_en.pdf. Accessed 18 September 2019.
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. . MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–20. - PubMed
-
- Russell ND, Marovich MA. Pox-Protein Public Private Partnership program and upcoming HIV vaccine efficacy trials. Curr Opin HIV AIDS 2016; 11:614–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069423/AI/NIAID NIH HHS/United States
- HHSN272201600012C/AI/NIAID NIH HHS/United States
- R21 MH083308/MH/NIMH NIH HHS/United States
- UM1 AI069481/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI154463/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- HHSN272201300033C/AI/NIAID NIH HHS/United States
- U01 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI069422/AI/NIAID NIH HHS/United States
- UM1 AI108568/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

