Frizzled 1 and Wnt1 as new potential therapeutic targets in the traumatically injured spinal cord
- PMID: 31900623
- PMCID: PMC11104978
- DOI: 10.1007/s00018-019-03427-4
Frizzled 1 and Wnt1 as new potential therapeutic targets in the traumatically injured spinal cord
Abstract
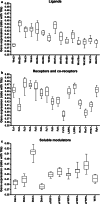
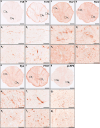
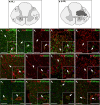
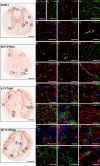
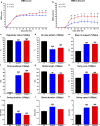
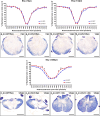
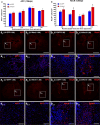
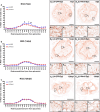
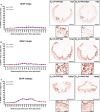
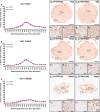
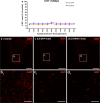
Despite the experimental evidence pointing to a significant role of the Wnt family of proteins in physiological and pathological rodent spinal cord functioning, its potential relevance in the healthy and traumatically injured human spinal cord as well as its therapeutic potential in spinal cord injury (SCI) are still poorly understood. To get further insight into these interesting issues, we first demonstrated by quantitative Real-Time PCR and simple immunohistochemistry that detectable mRNA expression of most Wnt components, as well as protein expression of all known Wnt receptors, can be found in the healthy human spinal cord, supporting its potential involvement in human spinal cord physiology. Moreover, evaluation of Frizzled (Fz) 1 expression by double immunohistochemistry showed that its spatio-temporal and cellular expression pattern in the traumatically injured human spinal cord is equivalent to that observed in a clinically relevant model of rat SCI and suggests its potential involvement in SCI progression/outcome. Accordingly, we found that long-term lentiviral-mediated overexpression of the Fz1 ligand Wnt1 after rat SCI improves motor functional recovery, increases myelin preservation and neuronal survival, and reduces early astroglial reactivity and NG2+ cell accumulation, highlighting the therapeutic potential of Wnt1 in this neuropathological situation.
Keywords: Frizzled 1; Human; Rat; Spinal cord injury; Wnt; Wnt1.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Spatio-temporal expression pattern of frizzled receptors after contusive spinal cord injury in adult rats.PLoS One. 2012;7(12):e50793. doi: 10.1371/journal.pone.0050793. Epub 2012 Dec 10. PLoS One. 2012. PMID: 23251385 Free PMC article.
-
Spatio-temporal and Cellular Expression Patterns of PTK7 in the Healthy and Traumatically Injured Rat and Human Spinal Cord.Cell Mol Neurobiol. 2020 Oct;40(7):1087-1103. doi: 10.1007/s10571-020-00794-6. Epub 2020 Jan 23. Cell Mol Neurobiol. 2020. PMID: 31974907 Free PMC article.
-
Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury.J Neurotrauma. 2014 Mar 15;31(6):565-81. doi: 10.1089/neu.2013.3067. J Neurotrauma. 2014. PMID: 24367909 Free PMC article.
-
Alpha-synuclein increases in rodent and human spinal cord injury and promotes inflammation and tissue loss.Sci Rep. 2021 Jun 3;11(1):11720. doi: 10.1038/s41598-021-91116-3. Sci Rep. 2021. PMID: 34083630 Free PMC article.
-
Enhancing motor functional recovery in spinal cord injury through pharmacological inhibition of Dickkopf-1 with BHQ880 antibody.Biomed Pharmacother. 2024 Jul;176:116792. doi: 10.1016/j.biopha.2024.116792. Epub 2024 May 24. Biomed Pharmacother. 2024. PMID: 38795645
Cited by
-
G protein-coupled receptors in neurodegenerative diseases and psychiatric disorders.Signal Transduct Target Ther. 2023 May 3;8(1):177. doi: 10.1038/s41392-023-01427-2. Signal Transduct Target Ther. 2023. PMID: 37137892 Free PMC article. Review.
-
Wnt1 oversees microglial activation by the Wnt/LRP5/6 receptor signaling pathway during lipopolysaccharide-mediated toxicity.Mol Biol Rep. 2025 Mar 1;52(1):273. doi: 10.1007/s11033-025-10360-2. Mol Biol Rep. 2025. PMID: 40025242 Free PMC article.
-
Cornerstone Cellular Pathways for Metabolic Disorders and Diabetes Mellitus: Non-Coding RNAs, Wnt Signaling, and AMPK.Cells. 2023 Nov 9;12(22):2595. doi: 10.3390/cells12222595. Cells. 2023. PMID: 37998330 Free PMC article. Review.
-
Silk-Elastin-like Polymers for Acute Intraparenchymal Treatment of the Traumatically Injured Spinal Cord: A First Systematic Experimental Approach.Pharmaceutics. 2022 Dec 3;14(12):2713. doi: 10.3390/pharmaceutics14122713. Pharmaceutics. 2022. PMID: 36559207 Free PMC article.
-
Wnt signaling pathway in spinal cord injury: from mechanisms to potential applications.Front Mol Neurosci. 2024 Jul 24;17:1427054. doi: 10.3389/fnmol.2024.1427054. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39114641 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

