Imaging of tumour response to immunotherapy
- PMID: 31900689
- PMCID: PMC6942076
- DOI: 10.1186/s41747-019-0134-1
Imaging of tumour response to immunotherapy
Abstract
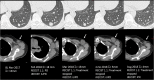
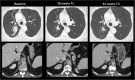
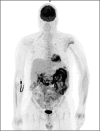
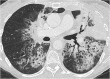
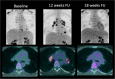
A wide range of cancer immunotherapy approaches has been developed including non-specific immune-stimulants such as cytokines, cancer vaccines, immune checkpoint inhibitors (ICIs), and adoptive T cell therapy. Among them, ICIs are the most commonly used and intensively studied. Since 2011, these drugs have received marketing authorisation for melanoma, lung, bladder, renal, and head and neck cancers, with remarkable and long-lasting treatment response in some patients. The novel mechanism of action of ICIs, with immune and T cell activation, leads to unusual patterns of response on imaging, with the advent of so-called pseudoprogression being more pronounced and frequently observed when compared to other anticancer therapies. Pseudoprogression, described in about 2-10% of patients treated with ICIs, corresponds to an increase of tumour burden and/or the appearance of new lesions due to infiltration by activated T cells before the disease responds to therapy. To overcome the limitation of response evaluation criteria in solid tumors (RECIST) to assess these specific changes, new imaging criteria-so-called immune-related response criteria and then immune-related RECIST (irRECIST)-were proposed. The major modification involved the inclusion of the measurements of new target lesions into disease assessments and the need for a 4-week re-assessment to confirm or not confirm progression. The RECIST working group introduced the new concept of "unconfirmed progression", into the irRECIST. This paper reviews current immunotherapeutic approaches and summarises radiologic criteria to evaluate new patterns of response to immunotherapy. Furthermore, imaging features of immunotherapy-related adverse events and available predictive biomarkers of response are presented.
Keywords: Cell- and tissue-based therapy; Immune checkpoint inhibitors; Immunotherapy; Pseudoprogression; Response evaluation criteria in solid tumors (RECIST).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
