Targeted deletion of PD-1 in myeloid cells induces antitumor immunity
- PMID: 31901074
- PMCID: PMC7183328
- DOI: 10.1126/sciimmunol.aay1863
Targeted deletion of PD-1 in myeloid cells induces antitumor immunity
Abstract
PD-1, a T cell checkpoint receptor and target of cancer immunotherapy, is also expressed on myeloid cells. The role of myeloid-specific versus T cell-specific PD-1 ablation on antitumor immunity has remained unclear because most studies have used either PD-1-blocking antibodies or complete PD-1 KO mice. We generated a conditional allele, which allowed myeloid-specific (PD-1f/fLysMcre) or T cell-specific (PD-1f/fCD4cre) targeting of Pdcd1 gene. Compared with T cell-specific PD-1 ablation, myeloid cell-specific PD-1 ablation more effectively decreased tumor growth. We found that granulocyte/macrophage progenitors (GMPs), which accumulate during cancer-driven emergency myelopoiesis and give rise to myeloid-derived suppressor cells (MDSCs), express PD-1. In tumor-bearing PD-1f/fLysMcre but not PD-1f/fCD4cre mice, accumulation of GMP and MDSC was prevented, whereas systemic output of effector myeloid cells was increased. Myeloid cell-specific PD-1 ablation induced an increase of T effector memory cells with improved functionality and mediated antitumor protection despite preserved PD-1 expression in T cells. In PD-1-deficient myeloid progenitors, growth factors driving emergency myelopoiesis induced increased metabolic intermediates of glycolysis, pentose phosphate pathway, and TCA cycle but, most prominently, elevated cholesterol. Because cholesterol is required for differentiation of inflammatory macrophages and DC and promotes antigen-presenting function, our findings indicate that metabolic reprogramming of emergency myelopoiesis and differentiation of effector myeloid cells might be a key mechanism of antitumor immunity mediated by PD-1 blockade.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
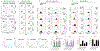

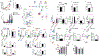

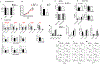



Comment in
-
A new perspective in cancer immunotherapy: PD-1 on myeloid cells takes center stage in orchestrating immune checkpoint blockade.Sci Immunol. 2020 Jan 3;5(43):eaaz8128. doi: 10.1126/sciimmunol.aaz8128. Sci Immunol. 2020. PMID: 31901075
-
Myeloid PD1 in the frame.Nat Rev Immunol. 2020 Feb;20(2):72-73. doi: 10.1038/s41577-020-0276-7. Nat Rev Immunol. 2020. PMID: 31937936 No abstract available.
-
PD1 Blockade in Cancer: Impact on Myeloid Cells.Trends Cancer. 2020 Jun;6(6):443-444. doi: 10.1016/j.trecan.2020.02.018. Epub 2020 Mar 13. Trends Cancer. 2020. PMID: 32459997
References
-
- Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T, A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nature immunology 14, 1212–1218 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

