Cortical Synaptic AMPA Receptor Plasticity during Motor Learning
- PMID: 31901303
- PMCID: PMC7060107
- DOI: 10.1016/j.neuron.2019.12.005
Cortical Synaptic AMPA Receptor Plasticity during Motor Learning
Abstract
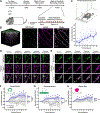
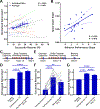
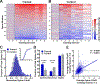
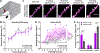
Modulation of synaptic strength through trafficking of AMPA receptors (AMPARs) is a fundamental mechanism underlying synaptic plasticity, learning, and memory. However, the dynamics of AMPAR trafficking in vivo and its correlation with learning have not been resolved. Here, we used in vivo two-photon microscopy to visualize surface AMPARs in mouse cortex during the acquisition of a forelimb reaching task. Daily training leads to an increase in AMPAR levels at a subset of spatially clustered dendritic spines in the motor cortex. Surprisingly, we also observed increases in spine AMPAR levels in the visual cortex. There, synaptic potentiation depends on the availability of visual input during motor training, and optogenetic inhibition of visual cortex activity impairs task performance. These results indicate that motor learning induces widespread cortical synaptic potentiation by increasing the net trafficking of AMPARs into spines, including in non-motor brain regions.
Keywords: AMPA receptors; long-term potentiation; motor cortex; motor learning; synaptic clustering; synaptic plasticity; two-photon imaging; visual cortex.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


Comment in
-
Eyes Wide Open on AMPAR Trafficking during Motor Learning.Neuron. 2020 Mar 4;105(5):764-766. doi: 10.1016/j.neuron.2020.02.006. Neuron. 2020. PMID: 32135087
References
-
- Bloss EB, Cembrowski MS, Karsh B, Colonell J, Fetter RD, and Spruston N (2018). Single excitatory axons form clustered synapses onto CA1 pyramidal cell dendrites. Nat. Neurosci 21, 353–363. - PubMed
-
- Bredt DS, and Nicoll RA (2003). AMPA receptor trafficking at excitatory synapses. Neuron, 40(2), pp.361–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

