Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity
- PMID: 31901900
- PMCID: PMC6977679
- DOI: 10.18632/aging.102646
Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity
Abstract
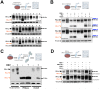
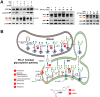
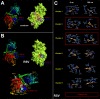
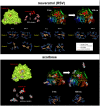
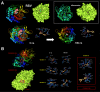
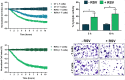
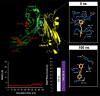
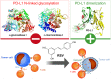
New strategies to block the immune evasion activity of programmed death ligand-1 (PD-L1) are urgently needed. When exploring the PD-L1-targeted effects of mechanistically diverse metabolism-targeting drugs, exposure to the dietary polyphenol resveratrol (RSV) revealed its differential capacity to generate a distinct PD-L1 electrophoretic migration pattern. Using biochemical assays, computer-aided docking/molecular dynamics simulations, and fluorescence microscopy, we found that RSV can operate as a direct inhibitor of glyco-PD-L1-processing enzymes (α-glucosidase/α-mannosidase) that modulate N-linked glycan decoration of PD-L1, thereby promoting the endoplasmic reticulum retention of a mannose-rich, abnormally glycosylated form of PD-L1. RSV was also predicted to interact with the inner surface of PD-L1 involved in the interaction with PD-1, almost perfectly occupying the target space of the small compound BMS-202 that binds to and induces dimerization of PD-L1. The ability of RSV to directly target PD-L1 interferes with its stability and trafficking, ultimately impeding its targeting to the cancer cell plasma membrane. Impedance-based real-time cell analysis (xCELLigence) showed that cytotoxic T-lymphocyte activity was notably exacerbated when cancer cells were previously exposed to RSV. This unforeseen immunomodulating mechanism of RSV might illuminate new approaches to restore T-cell function by targeting the PD-1/PD-L1 immunologic checkpoint with natural polyphenols.
Keywords: PD-L1; T cells; glycosylation; immunotherapy; resveratrol.
Conflict of interest statement
Figures

Similar articles
-
Molecular Mechanism of Food-Derived Polyphenols on PD-L1 Dimerization: A Molecular Dynamics Simulation Study.Int J Mol Sci. 2021 Oct 10;22(20):10924. doi: 10.3390/ijms222010924. Int J Mol Sci. 2021. PMID: 34681584 Free PMC article.
-
Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity.Nat Commun. 2016 Aug 30;7:12632. doi: 10.1038/ncomms12632. Nat Commun. 2016. PMID: 27572267 Free PMC article.
-
Tumor PD-L1 Induction by Resveratrol/Piceatannol May Function as a Search, Enhance, and Engage ("SEE") Signal to Facilitate the Elimination of "Cold, Non-Responsive" Low PD-L1-Expressing Tumors by PD-L1 Blockade.Int J Mol Sci. 2019 Nov 27;20(23):5969. doi: 10.3390/ijms20235969. Int J Mol Sci. 2019. PMID: 31783532 Free PMC article. Review.
-
The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment.Front Immunol. 2020 Oct 21;11:568931. doi: 10.3389/fimmu.2020.568931. eCollection 2020. Front Immunol. 2020. PMID: 33193345 Free PMC article. Review.
-
The impact of PD-L1 N-linked glycosylation on cancer therapy and clinical diagnosis.J Biomed Sci. 2020 Jul 3;27(1):77. doi: 10.1186/s12929-020-00670-x. J Biomed Sci. 2020. PMID: 32620165 Free PMC article. Review.
Cited by
-
Allergy Modulation by N-3 Long Chain Polyunsaturated Fatty Acids and Fat Soluble Nutrients of the Mediterranean Diet.Front Pharmacol. 2020 Aug 21;11:1244. doi: 10.3389/fphar.2020.01244. eCollection 2020. Front Pharmacol. 2020. PMID: 32973501 Free PMC article. Review.
-
Is the Triggering of PD-L1 Dimerization a Potential Mechanism for Food-Derived Small Molecules in Cancer Immunotherapy? A Study by Molecular Dynamics.Int J Mol Sci. 2023 Jan 11;24(2):1413. doi: 10.3390/ijms24021413. Int J Mol Sci. 2023. PMID: 36674929 Free PMC article.
-
ERK5 signalling pathway is a novel target of sorafenib: Implication in EGF biology.J Cell Mol Med. 2021 Nov;25(22):10591-10603. doi: 10.1111/jcmm.16990. Epub 2021 Oct 16. J Cell Mol Med. 2021. PMID: 34655447 Free PMC article.
-
Natural Polyphenols Targeting Senescence: A Novel Prevention and Therapy Strategy for Cancer.Int J Mol Sci. 2020 Jan 20;21(2):684. doi: 10.3390/ijms21020684. Int J Mol Sci. 2020. PMID: 31968672 Free PMC article. Review.
-
Metformin Is a Pyridoxal-5'-phosphate (PLP)-Competitive Inhibitor of SHMT2.Cancers (Basel). 2021 Aug 9;13(16):4009. doi: 10.3390/cancers13164009. Cancers (Basel). 2021. PMID: 34439169 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

